The Thyroid Gland, Parathyroid Glands, and Neck
Ambree Penrod
|
OBJECTIVES
Describe the thyroid gland embryology, surface anatomy, anatomic variants, and the common relational landmarks.
Discuss the physiology of the thyroid gland to include how each of the three thyroid hormones enables thyroid function.
Correlate laboratory values and clinical indications associated with hyperthyroidism and hypothyroidism.
Explain the sonographic evaluation of the thyroid gland to include patient preparation and protocol and demonstrate the examination procedure.
Differentiate normal and pathologic sonographic appearances associated with thyroid gland disease or pathology.
Describe the pathology, etiology, clinical signs and symptoms, and sonographic appearance for thyroid gland cysts, nodules, adenomas, goiters, thyrotoxicosis/hyperthyroidism, hypothyroidism, thyroiditis, thyroid disease in pregnancy, and thyroid carcinoma.
Explain the indications and guidelines for fine-needle aspiration.
Describe the parathyroid glands’ embryology, surface anatomy, anatomic variants, and the common relational landmarks.
Discuss the physiology of the parathyroid glands to include the importance of parathyroid hormone regulating calcium and phosphorus concentrations in extracellular fluid.
Correlate laboratory values and clinical indications associated with hypercalcemia and hypocalcemia.
Explain the sonographic evaluation of the parathyroid glands to include patient preparation and protocol and demonstrate the examination procedure.
Differentiate normal and pathologic sonographic appearances associated with disease or pathology of the parathyroid glands.
Describe the pathology, etiology, clinical signs and symptoms, and sonographic appearance for primary hyperparathyroidism to include adenomas, hyperplasia, and carcinoma.
Differentiate the varying sonographic appearances associated with normal anatomy and disease or pathology of the neck.
Describe the etiology, clinical signs and symptoms, and sonographic appearance of developmental cysts for the thyroglossal duct cyst, branchial cleft cyst, and cystic hygroma.
Identify the usefulness of diagnostic imaging to differentiate between a hematoma and deep neck space infections.
Describe the pathology, etiology, and important sonographic appearance and criteria to differentiate normal versus pathologic cervical lymph nodes.
KEY TERMS
anaplastic carcinoma
calcitonin (thyrocalcitonin)
elastography
euthyroid
follicular carcinoma
Graves disease
Hashimoto thyroiditis
Hürthle cell carcinoma
hypercalcemia
hyperparathyroidism
hyperplasia
hypocalcemia
hypothyroidism
medullary carcinoma
papillary carcinoma
subacute thyroiditis (de Quervain disease or granulomatous thyroiditis)
thyroiditis
thyrotoxicosis/hyperthyroidism
thyroxine (T4)
triiodothyronine (T3)
GLOSSARY
adenoma
parathyroid adenoma, a benign, solid tumor of the parathyroid gland that secretes parathyroid hormone, which results in elevated levels of serum calcium thyroid adenoma, a benign, solid tumor of the thyroid gland
parathyroid adenoma, a benign, solid tumor of the parathyroid gland that secretes parathyroid hormone, which results in elevated levels of serum calcium thyroid adenoma, a benign, solid tumor of the thyroid gland
adenopathy
enlargement of the glands
enlargement of the glands
anaplasia
a loss of differentiation of cells, which is a characteristic of tumor tissue and occurs in most malignant tumors
a loss of differentiation of cells, which is a characteristic of tumor tissue and occurs in most malignant tumors
cervical adenopathy
enlargement of the lymph nodes
enlargement of the lymph nodes
cold nodule (photon-deficient area)
seen on a nuclear medicine study as region of thyroid where the radioisotope has not been taken up; the area may correspond to a palpable mass
seen on a nuclear medicine study as region of thyroid where the radioisotope has not been taken up; the area may correspond to a palpable mass
euthyroid
state in which the thyroid gland is producing the right amount of thyroid hormone
state in which the thyroid gland is producing the right amount of thyroid hormone
fine-needle aspiration (FNA)
invasive procedure using a small gauge needle to obtain a tissue specimen from a specific lesion
invasive procedure using a small gauge needle to obtain a tissue specimen from a specific lesion
goiter
focal or diffuse thyroid gland enlargement often owing to iodine deficiency; multiple nodules may be present
focal or diffuse thyroid gland enlargement often owing to iodine deficiency; multiple nodules may be present
Graves disease
an autoimmune hyperthyroidism caused by antibodies that continuously activate thyroid-stimulating hormone receptors; it is characterized by enlarged thyroid, protrusion of eyeballs (exophthalmos), a rapid heartbeat, nervous excitability
an autoimmune hyperthyroidism caused by antibodies that continuously activate thyroid-stimulating hormone receptors; it is characterized by enlarged thyroid, protrusion of eyeballs (exophthalmos), a rapid heartbeat, nervous excitability
Hashimoto thyroiditis (chronic lymphocytic thyroiditis or Hashimoto disease)
most common inflammatory disease of the thyroid gland; usually occurs in genetically predisposed individuals, often presents in patients with other autoimmune disorders that may be associated with the formation of antibodies against normal thyroid tissue, and often accompanied by marked hyperemia
most common inflammatory disease of the thyroid gland; usually occurs in genetically predisposed individuals, often presents in patients with other autoimmune disorders that may be associated with the formation of antibodies against normal thyroid tissue, and often accompanied by marked hyperemia
heterotopic
occurring at an abnormal place or upon the wrong part of the body
occurring at an abnormal place or upon the wrong part of the body
hyperparathyroidism
disorder associated with elevated serum calcium levels; usually caused by benign parathyroid adenoma
disorder associated with elevated serum calcium levels; usually caused by benign parathyroid adenoma
hyperthyroidism
oversecretion of thyroid hormones
oversecretion of thyroid hormones
hypothyroidism
underactive thyroid hormones
underactive thyroid hormones
indolent
causing little pain (indolent tumor) or slow growing (indolent lesion or tumor)
causing little pain (indolent tumor) or slow growing (indolent lesion or tumor)
isthmus
thin band of thyroid tissue connecting the right and left lobes
thin band of thyroid tissue connecting the right and left lobes
longus colli muscles
wedge-shaped muscle posterior to the thyroid lobes
wedge-shaped muscle posterior to the thyroid lobes
microcalcifications
tiny hyperechoic foci that may or may not shadow; sometimes present within a thyroid nodule
tiny hyperechoic foci that may or may not shadow; sometimes present within a thyroid nodule
papillary carcinoma
most common form of thyroid cancer
most common form of thyroid cancer
parathyroid hormone
hormone produced by the parathyroid glands that regulate serum calcium and phosphorus
hormone produced by the parathyroid glands that regulate serum calcium and phosphorus
sternocleidomastoid muscles
large muscles located anterolateral to the thyroid
large muscles located anterolateral to the thyroid
strap muscles
sternohyoid and sternothyroid muscles located anterior to the thyroid
sternohyoid and sternothyroid muscles located anterior to the thyroid
thyroglossal duct cyst
developmental fluid-filled space; congenital anomaly located anterior to trachea extending from the base of the tongue to the isthmus of the thyroid
developmental fluid-filled space; congenital anomaly located anterior to trachea extending from the base of the tongue to the isthmus of the thyroid
thyroid inferno
increase in color Doppler vascular flow in the thyroid
increase in color Doppler vascular flow in the thyroid
thyroiditis
inflammation of the thyroid
inflammation of the thyroid
thyroid-stimulating hormone
hormone secreted by the anterior pituitary gland that stimulates the thyroid gland to secrete thyroxine (T4) and triiodothyronine (T3)
hormone secreted by the anterior pituitary gland that stimulates the thyroid gland to secrete thyroxine (T4) and triiodothyronine (T3)
Sonographic evaluation of the neck provides an important diagnostic screening procedure for the evaluation of both the thyroid gland and parathyroid glands, as well as the soft tissues of the neck. Using a high-resolution, high-frequency transducer, the noninvasive examination provides a fast and an accurate assessment of anatomy without patient preparation. Sonographic guidance is important during such interventional procedures as fine-needle aspiration (FNA) and alcohol ablation of adenomas of the parathyroid glands.1
THYROID GLAND
The thyroid gland is the site of synthesis, storage, and controlled secretion of thyroid hormones.2 It is the largest endocrine gland in the human body and functions to control the basal metabolic rate (BMR).3 With the development of high-resolution, high-frequency probes designed for scanning small parts, sonography has established itself as a superior modality for imaging the thyroid gland.4 Its greatest clinical value is in confirming mass location, differentiating between cystic and solid lesions, and imaging the biopsy needle during FNA. The thyroid gland is subject to an array of maladaptations, which are presented along with their sonographic appearance in this chapter.
Embryology
Emerging between the third and fourth gestational weeks, the thyroid gland is the earliest endocrine glandular structure to appear in the human embryo.5 The gland develops from an invagination in the floor of the primitive pharynx at the level of the first and second branchial arches, a point in the adult corresponding to the base of the tongue. This invagination is lined by cylindrical epithelial cells and can be distinguished at 16 to 17 days of gestation. These cells separate to form their pharyngeal connections by the fifth gestational week and migrate downward in front of the primitive pharynx and the developing hyoid bone. During this period of growth, the vesicle becomes a solid mass of epithelial cells and severs its connection with the pharyngeal cavity. This journey leaves behind a trace of epithelial cells known as the thyroglossal tract (duct), which normally solidifies and ultimately atrophies.6 Dividing into two lobes connected by an isthmus at 7 weeks, the thyroid gland forms a shield over the front of the trachea and thyroid cartilage, becoming fully developed by the end of the first trimester.6
Anatomy
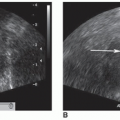
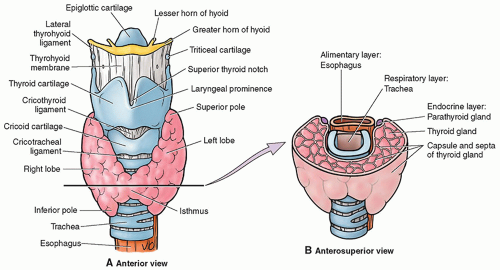
The thyroid gland is located in the anterior neck, surrounded by a fibrous capsule. It consists primarily of a right and left lobe, with a relatively thin isthmus, which unites the lobes usually over the second and third cartilaginous rings of the trachea.7 The superior border of the lateral lobes begins at approximately the thyroid cartilage (pomum Adami or Adam’s apple) and extends inferiorly (Fig. 17-1A, B).
The thyroid gland weighs approximately 30 g in the adult.3,8 The size and shape of the thyroid lobes vary with age, body surface area, and gender, being slightly larger in females.9 On longitudinal sections, the lobes appear elongated in tall individuals and appear more oval on short individuals.10 The mean length measures 40 to 60 mm, mean anteroposterior (AP) diameter is 13 to 18 mm, and the mean isthmic thickness is 4 to 6 mm.11 Sonography is very accurate for calculating thyroid gland volume when needed to determine treatment or evaluate response to treatment. The volume can be determined with linear measurements or mathematical formulas for each lobe,10 and vary by gender with female volumes ranging from 10 to 15 mL and males from 12 to 18 mL.3
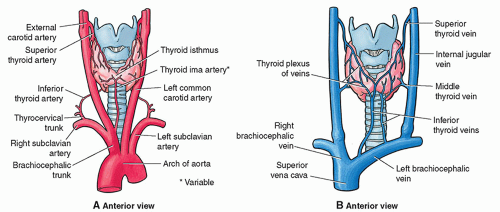
Four arteries provide a rich blood supply to the thyroid gland. The upper poles of the gland receive blood from paired superior thyroid arteries that arise from the external carotids. Two inferior thyroid arteries originate at the thyrocervical trunk of the subclavian artery and supply the lower thyroid poles12 (Fig. 17-2A). Normal peak velocities from the major thyroid arteries are 20 to 40 cm/sec and normal peak velocities from the intraparenchymal arteries are 15 to 30 cm/sec.10 On the anterior surface, three pairs of veins normally drain the thyroid plexus.12 The superior thyroid veins correspond to the superior thyroid artery and drain the superior lobes. The middle thyroid veins drain the middle lobes and the inferior thyroid veins drain the inferior poles. The superior and middle thyroid veins drain into the internal jugular vein and the inferior thyroid veins drain into the brachiocephalic veins12 (Fig. 17-2B).
Anatomic Variants
Deviations during any stage of development may lead to aberrant configurations or heterotopic locations of the thyroid gland. If the thyroglossal duct fails to involute completely, its persistence is a 1 to 3 cm cystic, fluid-filled remnant located anywhere along the route of the duct.6 The thyroglossal duct cyst is the most common congenital cyst found in the neck.13
The most critical abnormality is the absence of the thyroid gland (athyrosis). This rare condition is associated with cretinism or congenital hypothyroidism. Early identification and intervention with hormone replacement can stave off the physical and mental deficiencies associated with the congenital hypothyroidism.
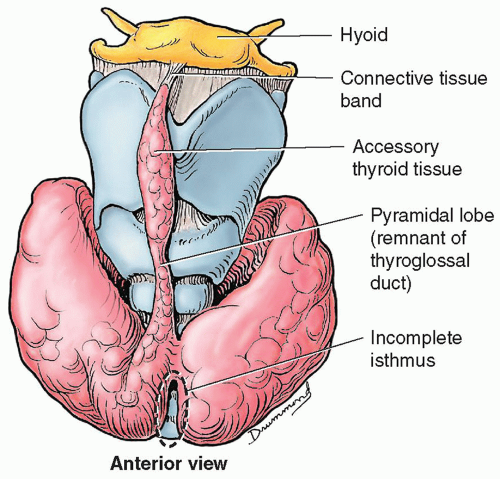
More commonly, the gland may differentiate into configurations other than an isthmus and two lateral lobes. The variation occurring most often is a pyramidal lobe, which has been identified to some degree in approximately as many as 50% of normal patients.5 Developmentally, the pyramidal lobe arises from the caudal portion of the thyroglossal tract. Usually, this lobe is small, extending midline upward from the isthmus, but it can arise from either lobe, more often the left lobe than the right12 (Fig. 17-3).
Other anatomic variations include the absence of an isthmus with the gland appearing as two independent lobes, the absence of one lobe with enlargement of the remaining lobe, or continuity from one lobe to the other effectively obliterating the isthmus.5
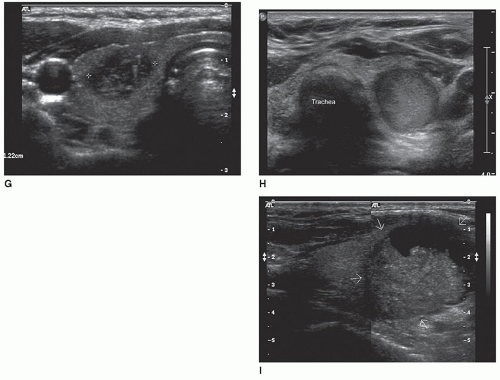
Thyroid gland development can occur ectopically at any point along the pathway of descent.12 A normal gland may also rest entirely above (suprahyoid or prelaryngeal) or below the hyoid bone. Lingual thyroid, although relatively rare (1 in 100,000 cases of thyroid disease), is the most common location for functional ectopic tissue.13 This placement is usually identified by an incidental finding of a mass at the back of the tongue.14
Other ectopic locations include under the tongue (sublingual), the mediastinum (substernal), and rarely
the tracheal or esophageal wall (Fig. 17-4). Small amounts of histologically functioning tissues may also be found along the internal carotid artery, the supraclavicular fossa, adjacent to the aortic arch or between the aorta and the pulmonary trunk, within the upper portion of the pericardium or mediastinum, and even within the interventricular septum.5,6,15
the tracheal or esophageal wall (Fig. 17-4). Small amounts of histologically functioning tissues may also be found along the internal carotid artery, the supraclavicular fossa, adjacent to the aortic arch or between the aorta and the pulmonary trunk, within the upper portion of the pericardium or mediastinum, and even within the interventricular septum.5,6,15
Anatomic Landmarks
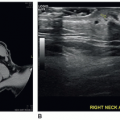
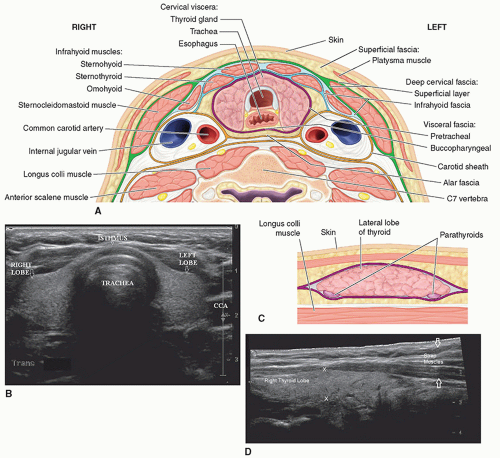
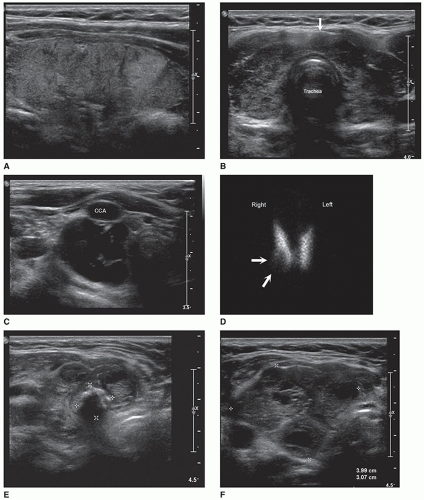
Many anatomic landmarks help to define the thyroid gland on a sonogram (Fig. 17-5A). On transverse images, the common carotid artery and internal jugular vein form the posterior lateral border of the gland. The artery is located medial to the vein. These structures are distinguished from the thyroid gland by echogenic walls and anechoic centers with color or pulsed wave Doppler imaging also available to assist in clarifying the anatomy. The longus colli muscle appears as a low-level, echogenic structure defining the posterior border of the gland. The air-filled trachea forms the medial border and appears hyperechoic, with posterior shadowing. The sternothyroid, sternohyoid, and omohyoid muscles, collectively called the strap muscles, form the anterolateral border of the gland. The sternothyroid muscle is directly superficial to the thyroid gland and is bordered by the sternohyoid anteriorly and the omohyoid laterally. The sternocleidomastoid is located lateral and superficial to the omohyoid. The very thin platysma muscle surrounds the neck, but its superficial location and indistinct density make it difficult to image with sonography. The thyroid gland appears as a rounded structure of low- to medium-level echoes, homogeneous in texture (Fig. 17-5B).
When imaged in the longitudinal (parasagittal) planes, the jugular vein and carotid artery appear as long, tubular, anechoic structures located laterally to the thyroid gland. Moving medially from these landmarks, the longus colli muscle, located posteriorly, becomes visible as a low-level, echogenic structure (Fig. 17-5C, D). The thyroid gland again is distinguished by its low- to medium-level, homogeneous echo pattern.
Physiology
Normal physical and mental growth depends on a healthy, functioning thyroid gland. The principal responsibility of the thyroid gland is maintenance of body metabolism.2 Protein, carbohydrate, lipid, and vitamin metabolism all rely on or are affected by thyroid hormone action. Additionally, thyroid hormones modulate oxygen consumption and enhance the rate of glucose uptake by fat tissue. Lipolysis and fatty acid mobilization from fat stores are magnified in the presence of thyroid hormone and blood serum cholesterol levels are generally lowered.3
The thyroid gland secretes three hormones: triiodothyronine (T3), thyroxine (T4), and calcitonin, also called thyrocalcitonin.2 The thyroid gland’s parafollicular cells, or C cells, secrete calcitonin, which lowers the plasma calcium level by inhibiting mobilization of calcium from the bone. The follicular cells of the thyroid gland chemically process iodine to secrete T3 and T4.8 The synthesis of these hormones depends on the availability of iodine and the gland’s ability to process it properly.3 When comparing secretion, the thyroid gland produces 90% of the less potent T4 and only 10% of the more potent T3; however, T4 is converted to the more powerful T3, which has the greatest metabolic effect and binds more efficiently to nuclear receptors in target cells.2
Maintenance of circulating concentrations of T3 and T4 is achieved by a dynamic regulatory system involving the hypothalamus, the pituitary, and the thyroid gland.2 Thyrotropin, secreted by the anterior pituitary (adenohypophysis) thyrotroph cells, orchestrates thyroid hormone production. The secretion of thyroid-stimulating hormone (TSH) is modulated by both the T3 and T4 hormones and by thyrotropin-releasing hormone (TRH) from the hypothalamus.2 Utilizing a classic negative feedback system, a drop in circulating thyroid hormones decreases the BMR. The falling BMR stimulates TRH, which in turn provokes the release of TSH. Thus inspired, the thyroid gland liberates the necessary T3 and T4, thereby returning the BMR to normal and retiring the cycle. Because the effects of thyroid hormones represent a complex integration of events at both the cellular and holistic levels, disease states that interfere with this system can have serious consequences.2
Laboratory Tests
Thyroid hormones circulate in the blood both free and bound to thyroxine-binding globulin (TBG).6 Thyroid gland with normal laboratory values is referred to as an euthyroid, which means the gland is producing the right amount of thyroid hormone. There are several tests for diagnosing thyroid disease, an example of which is given in (Table 17-1). The range of these values may vary somewhat among laboratories and in different geographic locations, and additional labs may be added to this list in different circumstances. For example, laboratory tests for calcitonin are done only for known or suspected cases of medullary carcinoma of the thyroid gland.
Diagnosing thyroid disease early is important because most are responsive to medical or surgical management.6 These include conditions associated with excessive release of thyroid hormones (hyperthyroidism), those associated with thyroid hormone deficiency (hypothyroidism), and mass lesions of the thyroid gland.6
TABLE 17-1 Thyroid Laboratory Values | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Sonographic Examination Technique
Sonography proves useful in a variety of situations when examining the neck. Sonographic examination can reliably distinguish between cystic and solid masses, provide guidance for FNA sampling or treatment of lesions,16 and establish pathology in the thyroid or neck following a normal clinical exam with nonpalpable lesions, as may be the case in patients with a history of therapeutic radiation to the head and neck. Serial sonographic examinations are also used to follow the size of suspected benign nodules on patients
receiving suppressive therapy or in those patients who require continued monitoring for growth of nodules. Sonographic exams aid in the determination of when further examination through biopsy or surgery may be necessary as is the case when nodules fail to decrease in size or continue to grow over a period of time. Additionally, sonography facilitates the treatment of several benign and malignant conditions through the use of sonographically guided percutaneous ethanol injection, which has been used as an alternative to surgery in patients not suitable for surgical intervention or in patients with recurrent cysts following FNA.16
receiving suppressive therapy or in those patients who require continued monitoring for growth of nodules. Sonographic exams aid in the determination of when further examination through biopsy or surgery may be necessary as is the case when nodules fail to decrease in size or continue to grow over a period of time. Additionally, sonography facilitates the treatment of several benign and malignant conditions through the use of sonographically guided percutaneous ethanol injection, which has been used as an alternative to surgery in patients not suitable for surgical intervention or in patients with recurrent cysts following FNA.16
To begin the examination, the medical history should be reviewed from the referring physician and the pertinent information obtained from the patient. Information about symptoms, duration, current treatment, history of therapeutic radiation, and locations of any palpable masses is necessary when interpreting the final images. If the patient has had a radioisotope scan or any prior imaging exams, sonographers should attempt to access and review the images and the associated reports because this will allow them to tailor their exam to attempt to answer any questions raised by preceding tests, or to follow up on indicated areas of concern. If the patient presents with a mass, either the patient should be made to identify its location or, if the mass is palpable only to the referring physician, a description of the area of interest should be obtained.
No patient preparation is required for thyroid gland sonography, although some facilities recommend limiting food and drink 1 hour prior to the examination to reduce gastric reflux in patients subject to this problem. With the patient in a supine position, the shoulders and upper back should be elevated with a pillow or rolled towel, hyperextending the neck.17 This will permit easier access to the thyroid gland. The sonographer should be cautious with elderly patients and other patients in whom this position may cause dizziness or neck strain and should not overextend the neck in any patient. If this position is not tolerable, the patient should be requested to elevate the chin up and back as far as possible, and, if needed, the patient’s head should be turned away from the side being examined in order to gain surface area for scanning.
The highest-frequency transducer available should be selected, remaining aware that the thyroid gland is a superficial structure and that near-field resolution will be important. Most current sonography equipment offer 7.5- to 15-MHz short-focus linear transducers specifically designed for small parts scanning. The movable focal zone (if applicable) should be adjusted to the area of interest in the gland. In patients with thick necks, or occasionally in those who have had radiation therapy, a lower-MHz transducer may be needed for penetration. Resolution is diminished at this lower frequency, which compromises structural clarity but may be necessary in these situations.
Scanning protocol includes multiple transverse, longitudinal, and oblique views. The sonographer should begin superiorly at the level of the mandible and then move inferiorly in the transverse plane until the characteristic pattern of the thyroid gland is identified. The homogeneously echogenic pattern results from the numerous follicles and surrounding supportive tissue that constitutes the thyroid gland. Sonographically, it is similar in appearance to normal parenchyma in the liver and testes and hyperechoic in appearance relative to adjacent musculature.7 The sonographer should stay alert for extra thyroid masses, such as enlarged lymph nodes or parathyroid glands, and proceed slowly through the gland and beyond, paying particular attention to subtle textural or structural changes. The procedure should be repeated for the opposite lobe. An attempt should be made to image both lobes and the isthmus on a single image with or without the use of wide screen settings. When a single image is not possible, most equipment will allow dual-screen imaging, which displays two images side by side. This feature provides a method for presenting both thyroid lobes and the connecting isthmus simultaneously when unable to fit the entire gland on a single image. Panoramic imaging is useful when scanning enlarged glands and, in some cases, may be the only way to demonstrate the thyroid gland in its entirety. The sonographer should record as many images as necessary to document normal or abnormal structures and at least acquire representative images from the upper, middle, and lower portions of both lobes, including the isthmus. Each lobe should be measured in both the AP and transverse planes, and the isthmus should be measured in the AP plane. Imaging of the lower poles can be enhanced by asking the patient to swallow, which momentarily raises the thyroid gland in the neck.10
To scan the longitudinal plane, the sonographer should begin lateral to the thyroid gland, imaging the carotid artery or jugular vein and move the transducer medially, again noting both the architecture of the gland and any extraglandular structures. The gland should be measured in its longest projection, using the dual-screen, wide-screen, or panoramic function if necessary. The examination should also be extended laterally to include the region of the carotid artery and jugular vein in order to identify enlarged cervical chain lymph nodes, superiorly to visualize submandibular adenopathy, and inferiorly to define any pathologic supraclavicular nodes.10 Color Doppler and pulsed wave Doppler imaging contribute to the thyroid gland sonography examination by revealing internal vascular detail. They are perhaps most helpful when examining ambiguous isoechoic or complex masses. The presence or absence of Doppler flow may help to differentiate among solid vascular masses, simple serous cysts with echogenic fluid, necrotic solid masses, and hemorrhagic cysts. When nodules are identified during an exam, their size should be measured as the thyroid gland was measured, in three dimensions, in longitudinal, anterior-to-posterior, and transverse planes, and their location within the gland should be documented along with any identifying characteristics of the nodule. If multiple nodules are noted, they should be numbered with their location documentation to allow for follow-up when it is required.
Pathology of the Thyroid Gland
The thyroid gland is host to benign, malignant, autoimmune, and metastatic conditions, all of which have varied and often overlapping sonographic appearances. Sonography is most useful in differentiating solid from cystic lesions and in patients in whom there is uncertainty about the origin of a neck mass. The sonography examination for thyroid gland disease should serve to amplify and clarify the clinical, laboratory, nuclear medicine, and cytopathology data obtained for a patient.
Cysts
Thyroid cysts are common in humans but the term is often used loosely to define thyroid disease.18 The true epithelium-lined cysts in the region of the thyroid gland
are uncommon and are almost always benign.13 Two true cysts are the thyroglossal duct cyst and the branchial cleft cyst, which can be differentiated from each other by their location. Thyroglossal duct cysts tend to be midline and branchial cleft cysts tend to be lateral to the carotids. More information regarding these two cysts in presented in the section on Developmental Cysts.
are uncommon and are almost always benign.13 Two true cysts are the thyroglossal duct cyst and the branchial cleft cyst, which can be differentiated from each other by their location. Thyroglossal duct cysts tend to be midline and branchial cleft cysts tend to be lateral to the carotids. More information regarding these two cysts in presented in the section on Developmental Cysts.
Benign nodular thyroid disease is common among adults and the prevalence increases with age.19 Sonographic evaluation indicates that 15% to 25% of these solitary thyroid nodules are either cystic or predominantly cystic and are sonographically described as either a mixed or a complex lesion.18,19 The etiology of the cystic portion of the thyroid nodule is usually hemorrhage or is subsequent degeneration of preexisting nodules.20 FNA cytology provides the diagnosis of benign versus malignant and cystic versus solid lesions.18 Percutaneous ethanol injection is used to initially treat benign cystic nodules or as follow-up treatment for recurrent thyroid cysts.19,20 The response rate to the ethanol injection ranges from 72.1% to 93.9%.18 Malignant cystic nodules are surgically removed.18
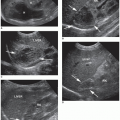
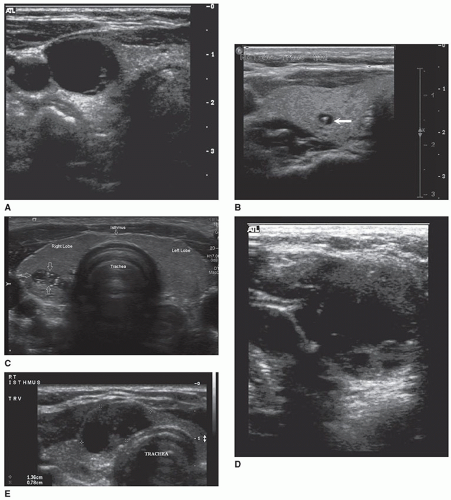
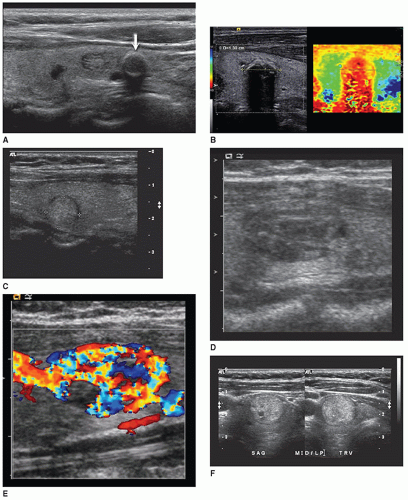
Sonographically, a simple cyst will be circular or oval, with discrete margins; contain no internal echoes; and exhibit posterior enhancement (Fig. 17-6A). Comet tail artifacts can frequently be encountered in complex cystic thyroid nodules, and they are likely related to the presence of colloid substances in the cyst21 (Fig. 17-6B). A hemorrhagic cyst may contain blood and debris and may appear as a complex mass with irregular borders and internal septa (Fig. 17-6C). When more densely echogenic fluid is gravitationally layered in the posterior portion of a cystic cavity, the likelihood of hemorrhagic debris is very high (Fig. 17-6D). Sonographically, papillary carcinomas may present with varying amounts of cystic change, may appear almost indistinguishable from benign cystic nodules, or may appear as uneven cystic structures, with finger-like pedunculated mass(es) larger than 2 cm seen projecting into the lumen18 (Fig. 17-6E). FNA is important to distinguish these from one another and to obtain a diagnosis.
Thyroid Nodule
In the United States, the estimated prevalence of thyroid nodules found by palpation alone ranges from 4% to 7% and sonography detects nodules in 20% to 76% of the adult population.22 They are more common in women and increase in frequency with age and with decreasing iodine intake.22 Based on the technetium-99m (Tc-99m) radioiodine scintigraphy examination, they are classified either as “hot” (hyperfunctioning/autonomous) or as “cold” (nonfunctioning).23 A cold nodule is one that does not absorb the radiopharmaceutical used for evaluating the gland and therefore appears as an area of decreased or absent activity on the resulting nuclear image. In contrast, a hot nodule traps an excessive amount of isotope and presents as a dense collection of activity.
Patients with a thyroid nodule palpated on physical examination, which subsequently appear as cold nodules on a nuclear medicine study, are often referred to sonography for further evaluation. Approximately 80% to 85% of thyroid nodules are cold and 10% to 15% of these are malignant.24 About 5% to 10% of solitary thyroid nodules are hot nodules, which usually implies benignity.23 Currently, no single sonographic criterion distinguishes benign thyroid nodules from malignant thyroid nodules with complete reliability.10 It is possible to make an accurate prediction of malignancy and recommend FNA when suspicious sonographic signs are seen in combination with multiple signs of thyroid malignancy.
Adenomas
A thyroid adenoma is a benign, neoplastic growth of thyroid glandular epithelium usually contained within a fibrous capsule.6 Most adenomas are solitary but they may also develop as part of a multinodular process.10 Although the terms adenoma and nodule are used interchangeably, an adenoma is a specific new tissue growth (neoplastic) and a nodule may include a carcinoma, a normal gland lobule, or any other focal lesion. Benign adenomas account for 5% to 10% of thyroid nodules and are seven times more common in females than in males.10 Most adenomas are derived from follicular epithelium, and a small minority of these are toxic and cause hyperthyroidism owing to autonomous function.13 Based on the degree of follicle formation and the colloid content of the follicles, the rare adenomas make up these histologic subtype classifications: macrofollicular (simple colloid), microfollicular (fetal), embryonal (trabecular), Hürthle cell (oxyphil, oncocytic) adenomas, atypical adenomas, and adenomas with papillae.8 Adenomas grow slowly, remain dormant for years, and are more common in the fifth and sixth decades of life.6 In order to be palpated on physical examination, an adenoma must reach a size of 0.5 to 1 cm. This explains why sonography has detected small nodules on a thyroid gland that was not detected on palpation. When a hyperfunctioning adenoma suppresses normal thyroid gland tissue, the normal tissue atrophies and the adenoma appears as a hot nodule against a background of minimal uptake on a radionuclide examination.6 A toxic hyperfunctioning adenoma may provoke thyrotoxicosis. The distinction between toxic and nontoxic adenomas cannot be made with sonography. Adenomas are typically asymptomatic but can grow large enough to exert pressure or develop hemorrhage, thus, becoming problematic.
The sonographic features of adenomas are influenced by the amount of structural degeneration and vary widely, appearing cystic to complex or solid. One other sonographic feature previously thought to indicate a benign nodule often associated with an adenoma is calcification along the rim, but malignant nodules may also have this appearance10 (Fig. 17-7A, B). The most common appearance is that of a solitary, well-circumscribed, oval or circular mass of variable size and echogenicity. Small, solid adenomas with uniformly low echogenicity can be mistaken for cysts. The distinction is made by noting the absence of through sound transmission behind a solid lesion. A peripheral hypoechoic-to-anechoic halo that completely or incompletely surrounds an adenoma is a relatively consistent finding. The halo, however, cannot be used as the only criterion and additional statistical information is necessary to establish the halo’s specificity.9 A halo in the nodule periphery may be seen with benign or malignant conditions and suggests that there is an acoustic interface that does not reflect the ultrasound across two different types of histology in the region of the benign or malignant nodule and the surrounding thyroid gland.25 In the case of an adenoma, this halo is thought to represent the fibrous capsule and the perinodal blood vessels, which can be seen by color Doppler imaging, and mild edema
or compressed normal thyroid parenchyma (Fig. 17-7C). Color Doppler imaging performed on an adenoma may have a “spoke and wheel” appearance with peripheral blood vessels extending toward the center of the lesion10 (Fig. 17-7D, E). Adenomas greater than 2.5 to 3 cm commonly display the sonographic characteristics of a complex cyst. These adenomatous cysts tend to have irregular shapes and borders with thickened walls, an incomplete capsule and are less sharply demarcated from surrounding tissue. Although imaging research is providing increased statistical probabilities to distinguish benign and malignant nodules, imaging procedures cannot be used as the only dependable criteria to distinguish adenomas from other benign or malignant nodules21 (Fig. 17-7F-I).
or compressed normal thyroid parenchyma (Fig. 17-7C). Color Doppler imaging performed on an adenoma may have a “spoke and wheel” appearance with peripheral blood vessels extending toward the center of the lesion10 (Fig. 17-7D, E). Adenomas greater than 2.5 to 3 cm commonly display the sonographic characteristics of a complex cyst. These adenomatous cysts tend to have irregular shapes and borders with thickened walls, an incomplete capsule and are less sharply demarcated from surrounding tissue. Although imaging research is providing increased statistical probabilities to distinguish benign and malignant nodules, imaging procedures cannot be used as the only dependable criteria to distinguish adenomas from other benign or malignant nodules21 (Fig. 17-7F-I).
Goiters
A nontoxic goiter is also termed simple, colloid, or multinodular and refers to an enlargement involving the entire gland without producing nodularity and without evidence of a functional disturbance.6 The enlarged follicles are filled with colloid.8 Nontoxic goiter occurs in both an endemic distribution with more than 10% of the population affected and a sporadic distribution.8 Endemic goiter occurs in geographic areas where the soil, water, and food supply contain low levels of iodine. The decrease or lack of iodine leads to decreased synthesis of thyroid hormone and a compensatory increase in TSH. Increased TSH levels lead to follicular cell hypertrophy, hyperplasia, and goitrous enlargement.8 Sporadic goiter is defined as a benign enlargement of the thyroid gland in euthyroid subjects living in an iodine-sufficient area. The sporadic goiter can be diffuse, uninodular, or multinodular.26 The cause of sporadic goiter is usually not apparent and may be related to ingestion of substances or hereditary enzymatic defects that interfere with thyroid hormone synthesis.8 The peak age of subjects with sporadic goiter is between 35 and 60 years and women are three times more likely than men to have the disease.10
Owing to recurrent episodes of hyperplasia and involution, simple goiters may convert into multinodular goiters. Nodularity of the thyroid gland can be the end stage of diffuse nontoxic goiter. As new follicles develop and outgrow their blood supply, hemorrhagic necrosis of all or part of the nodule results. Scarring then produces an inelastic network into which new follicles are squeezed, resulting in the formation of nodules.14 Calcifications, fibrosis, degenerative cysts, and hemorrhage result in the heterogeneous sonographic appearance. Multinodular goiters may be multilobulated with an asymmetrically enlarged gland. The pattern and location of enlargement are unpredictable and may involve only one lobe or may expand growing behind the sternum and clavicles to produce the intrathoracic or plunging goiters.8 Multinodular goiters may be nontoxic or may induce thyrotoxicosis (toxic multinodular goiters). As is the case of the simple goiter, the incidence of multinodular goiter is greater in females than in males.
The size of nontoxic goiters ranges from a doubling in size (40 g) to a massive enlargement in which the thyroid weighs a few hundred grams to more than 2,000 g.6,8 Symptoms caused by large goiters are usually associated with compressing the esophagus (dysphasia), trachea (inspiratory stridor), neck veins (venous congestion), or laryngeal nerve (hoarseness).6
The sonographic appearance may be nonspecific and varies with pathogenesis of the goiter. The visualization of a dominant nodule, a tender spot, or a region of focal hardness may provide pathologic clues25 (Fig. 17-8A, B). A second type of pathology may be suggested if one region in a goiter presents an echo pattern distinct from the rest of the goiter. It is important to note if there is a region within the goiter that displays sonographic features associated with increased risk
for malignancy, which include hypoechogenicity, solidity, microcalcification, irregular margin, and a taller-than-wide shape27 (Fig. 17-8C-F). Neoplasm and lymphomas have been demonstrated in goiters.25 Table 17-2 lists additional usefulness of sonographic examinations in goitrous patients.
for malignancy, which include hypoechogenicity, solidity, microcalcification, irregular margin, and a taller-than-wide shape27 (Fig. 17-8C-F). Neoplasm and lymphomas have been demonstrated in goiters.25 Table 17-2 lists additional usefulness of sonographic examinations in goitrous patients.
TABLE 17-2 Value of Sonographic Examination for Goitrous Patients25 | |
|---|---|
|
Thyrotoxicosis/Hyperthyroidism
Thyrotoxicosis is a hypermetabolic state caused by elevated levels of free T3 and T4.28 The terms thyrotoxicosis and hyperthyroidism are often used interchangeably because the condition is caused most commonly by hyperfunction of the thyroid gland.8 Hyperthyroidism is correct to use if elevated levels arise from hyperfunction, as occurs in Graves disease; thyrotoxicosis is correct to use if the increased hormone levels reflect excessive leakage of hormone out of a nonhyperactive gland.8 Primary hyperthyroidism is a form of thyrotoxicosis in which excess thyroid hormone is synthesized and secreted by the thyroid glands.8 Secondary hyperthyroidism is rare and is caused by TSH-secreting pituitary adenomas28 (Pathology Box 17-1).
Pathogenesis of either thyrotoxicosis or hyperthyroidism produces common clinical manifestations (Pathology Box 17-2). Children with Graves disease often have accelerated growth spurts and advanced bone age, symptoms of emotional lability, hyperactivity, difficulty concentrating, and occasionally failure to thrive.
The underlying cause of hyperthyroidism in 50% to 80% of cases is Graves disease.28 Graves disease is an autoimmune disease and about 75% of autoimmune diseases occur in women, most often during the childbearing years.29 Graves disease can occur at any age or gender but is most common in women of reproductive age.30 Research indicates a multifactorial etiology where different factors come together to cause Graves disease, such as heredity, the body’s immune system, age, gender, and possibly stress.29
Causes of Primary Hyperthyroidism
Graves disease
Toxic multinodular goiter
Solitary hyperfunctioning nodules
Follicular thyroid carcinoma (rare)
Thyroiditis
Ingestion of exogenous thyroid hormone administered for hypothyroidism
Causes of Secondary Hyperthyroidism
Secretion of excessive amounts of thyroid hormone by ectopic thyroid arising in ovarian teratomas (struma ovarii)
Cardiovascular system: increased cardiac output and decreased peripheral resistance; tachycardia at rest; loud heart sounds
Endocrine system: enlarged thyroid gland (goiter); hypercalcemia and decreased parathyroid hormone secretions
Gastrointestinal system: weight loss; increased peristalsis leading to diarrhea, nausea, vomiting, anorexia, abdominal pain
Integumentary system: excessive sweating, flushing, and warm skin; heat intolerance; temporary hair loss; palmar erythema
Musculoskeletal system: muscular weakness; children experience accelerated growth spurts and advanced bone age
Nervous system: nervousness; restlessness; short attention span; fatigue; fine hand tremor (particularly when outstretched); insomnia; increased appetite; emotional instability
Pulmonary system: dyspnea; reduced vital capacity
Reproductive system: oligomenorrhea or amenorrhea; erectile dysfunction and decreased libido
Sensory system (eyes): elevated upper eyelid (decreased blinking and staring feature); fine tremor of lid; variable eye changes
Graves disease is characterized as a multisystem syndrome consisting of one or more of the following: (1) hyperthyroidism, (2) diffuse thyroid enlargement (goiter), (3) ophthalmopathy (protrusion of the globe owing to fat accumulation and inflammation with edema), and (4) Graves dermopathy (pretibial myxedema characterized by subcutaneous swelling on the anterior portions of the legs and by indurated and erythematous skin).28
Thyrotoxicosis has a variety of causes, and determining the cause is important because treatment and expected outcomes will vary accordingly, though it will normally focus on controlling excessive thyroid hormone production.28 The diagnosis is based on symptoms of thyroid hormone excess and evaluating elevated serum-free T4 and T3 variances. TSH levels are increased in primary hyperthyroidism and decreased in secondary hyperthyroidism. Radioactive iodine uptake is a good diagnostic tool to determine the etiology of thyrotoxicosis.
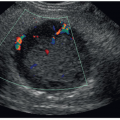
Sonography for thyrotoxicosis and hyperthyroidism can assess the size of the thyroid gland to facilitate decisions regarding treatment.25 Graves disease can present with either a normal-sized or an enlarged gland. When the gland is enlarged, the echo texture will usually be more heterogeneous compared to a diffuse goiter, which has numerous, large intraparenchymal vessels.10 Normal-sized glands will have a more uniform pattern. Hypervascularity is observed in most patients with Graves disease and has been quantified by the number of vessels per square centimeter measured on the greatest longitudinal view.31 The term “thyroid inferno” demonstrating multiple tiny areas of flow in the glandular tissue is often used to describe the hypervascular pattern
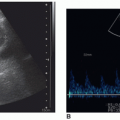
seen on color Doppler images (Fig. 17-9A-E). A bruit or thrill can often be heard over the gland. Spectral Doppler images may demonstrate peak velocities exceeding 70 cm/sec.10
seen on color Doppler images (Fig. 17-9A-E). A bruit or thrill can often be heard over the gland. Spectral Doppler images may demonstrate peak velocities exceeding 70 cm/sec.10
Hypothyroidism
Hypothyroidism is the most commonly occurring thyroid function disorder and affects between 0.1% and 2% of individuals in the United States.28 It is a clinical syndrome caused by a deficient production of thyroid hormone that results in reduced thyroid hormone action in the peripheral tissues. Hypothyroidism may be primary or secondary. There is a greater incidence and increased number of causes of primary hypothyroidism arising from an intrinsic abnormality in the thyroid gland (Pathology Box 17-3). Secondary (central) hypothyroidism occurs less frequently than primary hypothyroidism and includes those conditions that cause either pituitary or hypothalamic disease or damage resulting in failure to stimulate normal thyroid function.28 The conditions contributing to secondary hypothyroidism include pituitary adenoma; tumors impinging on the hypothalamus; irradiation; medication (dopamine, lithium); congenital disorders; and, rarely, Sheehan syndrome.
Defective hormone synthesis
Autoimmune disease (Hashimoto thyroiditis most common); postpartum thyroiditis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




 PATHOLOGY BOX 17-1
PATHOLOGY BOX 17-1  PATHOLOGY BOX 17-2
PATHOLOGY BOX 17-2 
 PATHOLOGY BOX 17-3
PATHOLOGY BOX 17-3