The Pediatric Abdomen
Tara K. Cielma
Anjum N. Bandarkar
|
OBJECTIVES
Demonstrate the sonographic scanning techniques, technical considerations, and routine examination for the neonatal and pediatric abdomen to include the prevertebral vessel (aorta and inferior vena cava) evaluation, liver, gallbladder and biliary system, pancreas, gastrointestinal tract, and retroperitoneum.
Describe the pathology, etiology, and clinical signs and symptoms for anomalies and pathology of the aorta and inferior vena cava, liver, gallbladder and biliary system, pancreas, gastrointestinal tract, and retroperitoneum in the neonate and pediatric patient.
Differentiate between the sonographic appearance of the normal prevertebral vasculature and the sonographic appearance for congenital anomalies and acquired pathology of the prevertebral vessels, liver, gallbladder and biliary system, pancreas, gastrointestinal tract, and retroperitoneum in the neonate and pediatric patient.
Identify technically satisfactory and unsatisfactory sonographic examinations of the abdomen on the neonatal and pediatric patient.
List the indications for the sonographic evaluation of urinary system and adrenal glands in the pediatric patient.
Explain the protocol process for sonographic evaluation of the urinary system and adrenal glands in the pediatric patient.
Identify the normal sonographic appearance of the urinary system and the adrenal glands in the pediatric patient.
Describe the pathology, etiology, clinical signs and symptoms, and sonographic appearance of common congenital abnormalities, tumors, and acquired pathology in the upper and lower urinary system in the pediatric patient.
Discuss three criteria for sonographic documentation of tumors on pediatric patients to include (1) origin of the mass, (2) extent of the mass, and (3) metastases.
Describe the pathology, etiology, clinical signs and symptoms, and sonographic appearance of congenital abnormalities, tumors, hemorrhage, cysts, and abscesses of the adrenal glands in the pediatric patient.
Identify technically satisfactory and unsatisfactory sonographic examinations of the urinary system and adrenal glands on the neonatal and pediatric patient.
KEY TERMS
adrenal hemorrhage
adrenocortical carcinoma
angiomyolipoma
appendicitis
biliary atresia
Budd-Chiari syndrome
Caroli disease
cavernous hemangioma
cholecystitis
choledochal cyst
cholelithiasis
cirrhosis
congenital adrenal hyperplasia
Crohn disease
cystic fibrosis
cystitis
duplicated collecting system
echinococcal cyst
glomerular cystic disease
hemangioendothelioma
hepatic fibrosis
hepatitis
hepatoblastoma
hepatocellular carcinoma
hepatoma
hydronephrosis
intussusception
juvenile nephronophthisis
medullary sponge kidney
mesenchymal hamartoma
mesenchymal sarcoma
mesoblastic nephroma
multicystic dysplastic kidney
multilocular cystic nephroma
nephroblastomatosis
nephrocalcinosis
neuroblastoma
pancreatic carcinoma
pancreatitis
pheochromocytoma
polycystic kidney disease
posterior urethral valves
portal hypertension
pseudocyst
pyloric stenosis
renal agenesis
renal cyst
renal dysplasia
renal hypoplasia
rhabdomyosarcoma
sacrococcygeal teratoma
sclerosing cholangitis
urachal cyst
ureterocele
Wilms tumor
GLOSSARY
AFP
alpha-fetoprotein; a tumor marker frequently elevated in cases of hepatocellular carcinoma, hepatoblastoma, and certain testicular cancers
alpha-fetoprotein; a tumor marker frequently elevated in cases of hepatocellular carcinoma, hepatoblastoma, and certain testicular cancers
biloma
a walled-off collection of bile caused by a disruption of the biliary tree, frequently caused by trauma or surgical procedures
a walled-off collection of bile caused by a disruption of the biliary tree, frequently caused by trauma or surgical procedures
coarctation
a narrowing or constriction
a narrowing or constriction
enuresis
involuntary discharge of urine
involuntary discharge of urine
hemobilia
hemorrhage or blood in the bile caused by bleeding into the biliary tree
hemorrhage or blood in the bile caused by bleeding into the biliary tree
hemoperitoneum
blood in the peritoneal cavity
blood in the peritoneal cavity
hyperalimentation
the administration of nutrients through intravenous feeding
the administration of nutrients through intravenous feeding
hyponatremia
an electrolyte imbalance; low sodium levels in the blood
an electrolyte imbalance; low sodium levels in the blood
ileus
failure of the normal propulsion of the digestive tract
failure of the normal propulsion of the digestive tract
jaundice
yellowish pigmentation of the skin and whites of the eyes caused by increased levels of bilirubin in the blood
yellowish pigmentation of the skin and whites of the eyes caused by increased levels of bilirubin in the blood
reflux
occurs when valves at the junction of the ureter and bladder work incorrectly and allow urine from the bladder to back up into the ureter and kidney
occurs when valves at the junction of the ureter and bladder work incorrectly and allow urine from the bladder to back up into the ureter and kidney
ureteropelvic junction
area where the renal pelvis connects to the ureter
area where the renal pelvis connects to the ureter
Sonography is the noninvasive modality of choice to evaluate the neonatal and pediatric abdomen owing to the lack of ionizing radiation, the portability of the equipment, and excellent visualization of the abdominal anatomy in this age group. Critically ill patients who are sensitive to stress (i.e., transport, temperature changes) can easily and safely be examined at the bedside. Pediatric sonography presents many opportunities as well as challenges. Childhood obesity is a serious health care problem, and obese children may be as challenging to examine sonographically as adults. Obese children may also present with some disease processes previously seen only in adults, so the sonographer must have an in-depth knowledge of both pediatric and adult pathology.
Scanning the pediatric age group will require sonographic equipment with a wide range of probe frequencies. Depending on the area or organ of interest, a high-frequency (7 to 10 MHz) curved or (8 to 11 MHz) micro-convex or (8 to 15 MHz) linear-array transducer is utilized. Scanning infants and small children requires the sonographer to be adept at assessing the anatomy and acquiring images quickly. Distraction techniques are used for this age group rather than sedation. Children with high level of pain, anxiety, or autism spectrum disorder with comorbid developmental delay may or may not be able to cooperate fully during the
sonogram, so distractions such as headphones, development-appropriate movies, or other resources can be used to ensure the sonographer can complete the examination accurately and in a timely manner. Many health care facilities now employ Certified Child Life Specialists; these professionals may be utilized to assist with therapeutic play and assist with coping skills to manage stressful experiences.
sonogram, so distractions such as headphones, development-appropriate movies, or other resources can be used to ensure the sonographer can complete the examination accurately and in a timely manner. Many health care facilities now employ Certified Child Life Specialists; these professionals may be utilized to assist with therapeutic play and assist with coping skills to manage stressful experiences.
A parent or legal guardian will most likely be present for the examination. The sonographer must be prepared to professionally interact with the parent and elicit their assistance with the examination, as needed. The sonographer should always explain the examination to the patient using age-appropriate terms, provide realistic expectations, and should answer the parent’s questions about the examination in accordance with department policies and procedures. Special precautions should be taken to keep infants warm by placing blankets over all but the scanning surface. Warm gel should always be used on children. Single packets of gel for infection control must be used for neonates and critical care patients. Sterile gel packets should be used whenever a sterile area must be maintained or in cases where infection is of high concern. The sonographer must always follow infection control standards when scanning, and this can be even more important when examining pediatric patients.
A pediatric abdominal sonogram should include an assessment of all the organs, structures, and vessels of the abdomen. This chapter discusses the abdominal vessels as well as the liver, gallbladder/biliary system, pancreas, gastrointestinal (GI) tract, retroperitoneum, urinary system, and adrenal glands. The sonographer should have as much information as possible regarding the reason for the sonogram, incorporate prior diagnostic imaging, and be prepared to adapt the examination to the patient’s condition and any sonographic findings.
Patient Preparation
Patient preparation will vary based on the age of the patient and the area or organ of interest. Ideally, the liver and biliary tree are best viewed with the patient in a fasting state. As infants are fed every 3 to 4 hours, the examination should be performed just before a feeding. Children aged 1 to 3 years are best examined 4 hours after fasting and older children 6 hours after fasting. Children with gastronomy (G) or gastronomy-jejunostomy (GJ) tubes are typically fasted between 4 and 6 hours before the procedure. Diabetic patients may require prioritization according to their insulin schedule and may be permitted clear liquids.
PREVERTEBRAL VESSEL EVALUATION
Sonographic Examination Technique
Patient Preparation
Although anatomically they are similar to adults, neonates and children require a different scanning approach. Using multiple planes on the neonate, the full length of the great vessels can easily be evaluated from the level of the diaphragm to the bifurcation without any particular patient preparation.
Depending on which great vessel is to be evaluated, it may be necessary to turn the patient to the appropriate side. Patients in the neonatal intensive care unit are often intubated, so if it is necessary to turn the patient onto one side or the other, it is advisable to seek the aid of the bedside nurse.
Scan Technique
In the neonate, coronal scanning is often most effective in demonstrating the aorta and inferior vena cava (IVC). Scanning from a right coronal approach is more optimal for evaluating the IVC because the vessel is closer to the transducer placed on the right lateral abdomen. Similarly, a left coronal approach on the left lateral abdomen is used to visualize the aorta.
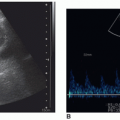
The sonographic examination of the abdominal vessels should include assessment of the vessels in multiple scan planes and the use of color and spectral Doppler to assess blood flow. The sonographer should acquire documentary images that clearly demonstrate the aorta and IVC from proximal to distal (bifurcation), including sonographically visible branches and tributaries. Split-screen color Doppler and grayscale imaging can be used to demonstrate the vessels when grayscale alone is insufficient, or pathology is present. Color and spectral Doppler should also be used to evaluate flow in the aorta, IVC, right and left iliac arteries and veins, and right and left renal arteries and veins. Correct presentation of the abdominal aorta and IVC must be documented so that the correct location and course of these vessels are confirmed.
When present in the neonate, an indwelling catheter in the aorta and its relationship to the renal arteries should be demonstrated. Although an umbilical arterial catheter (UAC) is visible on a radiograph, the location relative to the origin of the renal arteries cannot be reliably determined. Proper location of the tip of the UAC is in the aorta well above the level of the renal arteries. Umbilical venous catheter (UVC) may also be present in the umbilicus next to a UAC (or by itself). The UVC should course through the liver via the umbilical vein and the left hepatic vein. The tip should be in the proximal IVC near the junction of the right atrium. Sonographically, the UAC and UVC are visualized as hyperechoic parallel lines with an anechoic center. Shadowing from the walls of the line may be noted when the beam is perpendicular to the catheter. It is important not to confuse this for intrahepatic calcifications, which may result as a complication from improper UVC placement.
Normal Anatomy
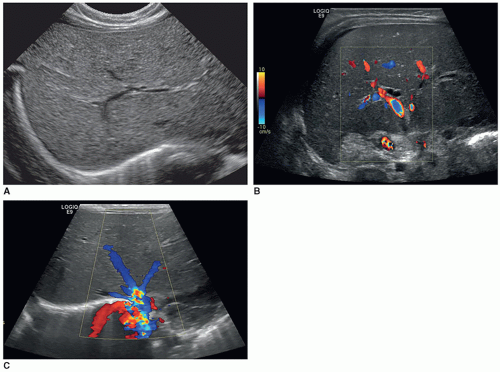
The sonographic appearance of the abdominal vessels in the pediatric patient is the same as in an adult (Fig. 20-1A, B). The vessels should have anechoic lumens with hyperechoic walls. The walls of the abdominal aorta may appear more echogenic than the walls of the IVC. The normal spectral Doppler of the aorta shows a pulsatile vessel with a high-resistance flow pattern (rapid upstroke, sharp systolic peak, and low-flow velocity with a small amount of reversed flow possible during diastole). The normal spectral Doppler flow pattern of the IVC is monophasic.
Congenital Anomalies
Hypoplasia or coarctation of the abdominal aorta is a rare congenital defect. The proximal descending thoracic aorta is affected in 98% of coarctations, only 2% of them actually affect the abdominal aorta (Fig. 20-2A, B). Renal artery stenosis occurs in more than half of abdominal coarctations. Congenital abdominal coarctation can occur at any time in embryonic development. The earlier it occurs, the more obvious the manifestations. Acquired coarctation of the abdominal aorta
has been associated with hypercalcemia, neurofibromatosis, tuberous sclerosis, rubella, and Turner syndrome. Children present with severe hypertension, headaches, and fatigue, whereas infants exhibit failure to thrive. An interrupted abdominal aorta produces vascular compromise with symptoms such as cyanotic, mottled, and discolored limbs with decreased femoral pulses. The extreme consequences of untreated severe hypertension can be fatal by the age of 30 years.
has been associated with hypercalcemia, neurofibromatosis, tuberous sclerosis, rubella, and Turner syndrome. Children present with severe hypertension, headaches, and fatigue, whereas infants exhibit failure to thrive. An interrupted abdominal aorta produces vascular compromise with symptoms such as cyanotic, mottled, and discolored limbs with decreased femoral pulses. The extreme consequences of untreated severe hypertension can be fatal by the age of 30 years.
Inferior Vena Cava
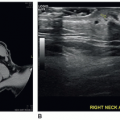
When scanning the IVC and the aorta, the sonographer must note both the position and relationship of the two vessels. In the normal relationship, the IVC is located on the right side, receiving the hepatic veins as it enters the right atrium. An IVC on the patient’s left side is diagnostic of situs inversus. Besides an abnormal relationship of the IVC and aorta, the IVC can also be interrupted, in which case it drains via an azygous continuation, which may lie on either the left or the right of the spine. The hemiazygous continuation lies more posterior than the aorta (Fig. 20-3A, B). Another abnormal vessel that may be imaged in the long-axis plane is an anomalous venous connection associated with total anomalous pulmonary venous return, which connects to the ductus venosus. It crosses between the aorta and IVC. Displacement or distortion of the IVC or the aorta should alert the sonographer that other anomalies may be present. Sonographers must be cognizant of the fact that unusual presentations of the aorta or IVC and anomalous vessels in the lower abdomen may indicate complex congenital heart disease.
Abdominal Aortic Thrombosis in the Neonate
The most common reason for evaluating the aorta in the neonate is for aortic thrombus, a well-recognized complication of indwelling UACs. Clinical signs of aortic thrombus include absent femoral pulses, hematuria, cyanosis, hypertension, blanching of the lower extremities, and necrotizing enterocolitis (NEC). An overly distended urinary bladder may cause some of the abovementioned symptoms.
Suspected aortic thrombus should be evaluated by a thorough scan of the entire aorta and both kidneys in multiple planes. Thrombus typically appears sonographically as echogenic material within the aortic lumen, which may totally or partially fill the vessel. The clot may be long and thick and is termed extensive if it fills 40% of the aorta in a sectional plane, goes to the level of the renal artery or iliac artery, or causes proximal dilatation. As the sonographic appearance of thrombus changes over time, the vessel may appear to contain thin linear structures. Color Doppler should be used to demonstrate any blood flow around the thrombus, normal flow reversal, and the presence of any collateral vessels. Grayscale and color Doppler should be used to follow the progression and/or resolution of the thrombus (Fig. 20-4A-D).
Inferior Vena Cava Thrombosis8
The IVC can be a site of thrombus or calcifications in neonates. IVC thrombosis can also occur secondary to indwelling catheters (such as UVC), clotting disorders, dehydration, sepsis, nephrotic syndrome, and extension of renal and/or pelvic vein thrombosis (Fig. 20-5A, B).
Inferior Vena Cava Tumor Invasion8
Children can have tumor invasion into the IVC, from Wilms tumor (Fig. 20-5C-E). Tumor extension can occur from the kidney, adrenal gland (neuroblastoma), retroperitoneum (sarcoma), and from hepatocellular carcinoma (HCC), teratoma, and lymphoma. It is important to evaluate the
extension of the tumor into the hepatic veins or right atrium and to seek evidence of tumor invasion into the wall of the IVC. Tumor extension appears similar to the solid texture of the tumor itself. The differential diagnosis includes simple thrombus. Computed tomography (CT) is the modality of choice for evaluating IVC wall invasion; however, sonography is the best modality for evaluating cephalad extension of IVC tumor invasion.
extension of the tumor into the hepatic veins or right atrium and to seek evidence of tumor invasion into the wall of the IVC. Tumor extension appears similar to the solid texture of the tumor itself. The differential diagnosis includes simple thrombus. Computed tomography (CT) is the modality of choice for evaluating IVC wall invasion; however, sonography is the best modality for evaluating cephalad extension of IVC tumor invasion.
LIVER9
When imaging the neonatal or pediatric liver, it is important to image the same landmarks as in an adult examination. Special attention should be paid to the liver parenchyma, the position and size of the gallbladder, portal vein, portal vein bifurcation, hepatic artery, common bile duct, and hepatic veins.
Sonographic Examination and Technique
Scan Technique
Establishing a protocol of longitudinal, coronal, and transverse planes is important to ensure consistency from one patient to the next; however, special attention must be paid to differences, such as position of anatomy, pathology, and size. The sonographic examination of the pediatric liver should include assessment of the liver parenchyma, vessels and ligaments in multiple scan planes, and the use of color and/or spectral Doppler to assess blood flow. Patients are most commonly scanned in the supine position, but it is beneficial to utilize a left posterior oblique (LPO) position in older children and/or those with a larger body habitus. After sweeping through the entire liver, the sonographer should acquire representative images that clearly demonstrate the lobes and segments of the liver, including vascular and ligament landmarks. The sonographer should be careful to assess and document the periphery of the liver as well as the bulk of the liver; longitudinal and transverse sweeps should extend past the lateral, superior, and inferior borders of the liver. Normal measurement parameters for the liver have been reported and show correlation with age, height, and weight. Longitudinal images demonstrating the lower pole of the right kidney in relationship to the inferior margin of the liver can be helpful.
Normal Anatomy
The normal liver appears as a smooth-outlined, homogeneous organ, usually situated in the right upper quadrant of the abdomen (Fig. 20-6A). The neonatal liver may appear mildly hyperechoic. The liver is divided into a large right lobe in the right side of the abdomen, a smaller left lobe extending across the midline, a caudate lobe situated on the posterior
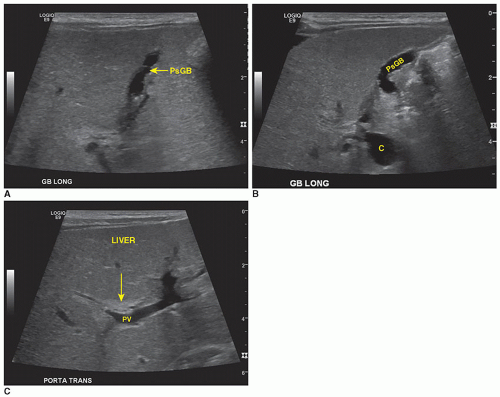
superior surface of the right lobe, and a quadrate lobe on the posteroinferior surface of the right lobe. The falciform ligament divides the right and left lobes. The liver receives a dual blood supply. The liver receives oxygenated blood from the hepatic artery, a branch of the celiac artery. Additional blood from the digestive system is carried to the liver via the portal vein, formed by the convergence of the superior mesenteric vein (SMV) and the splenic vein. The portal vein enters the liver at the porta hepatis, where it quickly branches into the right and left portal veins. The hepatic artery also enters the liver at the porta hepatis (Fig. 20-6B). The major vessels of the liver provide important visual and anatomic landmarks (Fig. 20-6C).
superior surface of the right lobe, and a quadrate lobe on the posteroinferior surface of the right lobe. The falciform ligament divides the right and left lobes. The liver receives a dual blood supply. The liver receives oxygenated blood from the hepatic artery, a branch of the celiac artery. Additional blood from the digestive system is carried to the liver via the portal vein, formed by the convergence of the superior mesenteric vein (SMV) and the splenic vein. The portal vein enters the liver at the porta hepatis, where it quickly branches into the right and left portal veins. The hepatic artery also enters the liver at the porta hepatis (Fig. 20-6B). The major vessels of the liver provide important visual and anatomic landmarks (Fig. 20-6C).
Applicable laboratory tests include the standard liver function tests and alpha-fetoprotein (AFP), which, if elevated, may indicate the presence of a hepatoblastoma or other malignant tumor.
Congenital Anomalies and Benign Tumors
Hemangiomas
Biliary atresia is a congenital anomaly that intricately involves the liver and is discussed in “Gallbladder and Biliary System” section. Other congenital anomalies of the liver are primarily composed of benign tumors.
Hemangiomas of the liver are congenital anomalies arising from an arteriovenous malformation, forming blood-filled spaces. They are the most common vascular liver tumor in infancy and are either cavernous (blood-filled spaces lined with a single layer of endothelial cells) or hemangioendotheliomas (the lining or endothelium is multilayered or hypertrophic, with primitive or infantile cells).
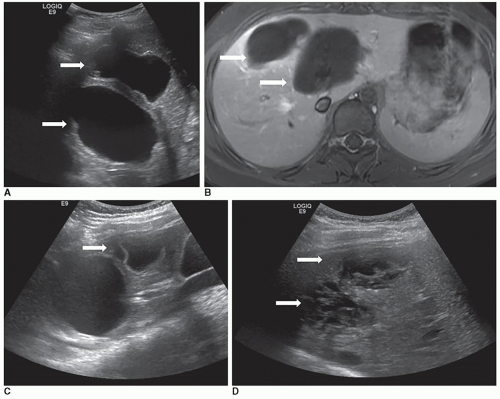
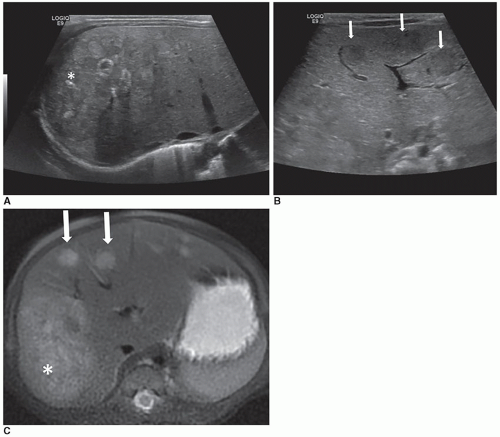
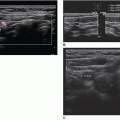
Infantile hepatic hemangiomas usually affect infants less than 6 months of age, are typically multiple, and are associated with cutaneous hemangiomas. Patients are typically symptomatic and present clinically with hepatomegaly, congestive heart failure, and hemoperitoneum from rupture. Sonographically, the lesions can appear hypoechoic, isoechoic, or hyperechoic to adjacent liver tissue, homogeneous or complex, and may contain echogenic foci (Fig. 20-7A-F).
Depending on the composition, variable acoustic enhancement may be present.
Hemangiomas are three times more common in girls than in boys. They may or may not be present at birth but usually become evident at about 2 months of age, or they may be found incidentally. Large hemangiomas may cause hepatomegaly, with or without accompanying abdominal distention. Hemangiomas cease to grow and then undergo spontaneous involution.
After the tumor enlarges but before regressing, the infant may experience a number of complications, including fatal rupture of the hemangioma, Kasabach-Merritt syndrome due to platelet trapping, hepatic dysfunction due to portal hypertension, intravascular coagulation, intestinal bleeding, bowel obstruction, obstructive jaundice, and irreversible congestive heart failure, as well as respiratory insufficiency caused by the mass effect.
Typical sonographic findings are of a well-defined, hyperechoic area within the liver (Fig. 20-8A). The hyperechoic appearance of hemangioma results from the multiple interfaces between the walls of the blood-filled sinuses. Less frequently, the mass is hypoechoic and may mimic a collection of simple cysts. It can also appear complex, demonstrating irregular walls and hypoechoic to anechoic areas, possibly due to necrosis (Fig. 20-8B, C). Contrast-enhanced ultrasound may be used to delineate perfusion patterns (Fig. 20-8D).
The presence of calcifications or fibrotic changes within the mass produces a hyperechoic pattern with posterior acoustic shadowing. Doppler interrogation may reveal high flow within the lesion. An enlarged hepatic artery, as well as a small distal aorta due to the increased hepatic flow, can also be seen.
Needle biopsy for confirmation of hemangiomas is a dangerous procedure that can result in fatal hemorrhage, so it is usually used as a last resort when a diagnosis cannot be reached by other means. The cytologic diagnostic criteria include the presence of benign epithelial cells, fresh blood from the mass, and no malignant cells.
The treatment of hemangioma varies with the size of the mass. Most hemangiomas undergo spontaneous involution and regression, but when a large hemangioma threatens the patient’s health, aggressive procedures are instigated. A lobectomy or resection of the tumor is sometimes performed, but if the patient is experiencing congestive heart failure, hepatic artery ligation or embolization can be performed. In many cases, the lesion is responsive to steroid therapy and radiation therapy.
The differential diagnosis includes angiomatous tumors, hepatoblastoma, hepatoma and metastatic neuroblastoma, cysts, abscesses, and focal nodular hyperplasia.
Mesenchymal or fibrous hamartoma is a rare congenital anomaly that arises from the connective tissue or mesenchyme of the portal tracts. It is considered to be the second most common benign hepatic mass seen in children and is more common in males.
The lesion usually presents within the first 2 years of life, with painless abdominal swelling and anorexia as the first clinical symptoms. Congestive heart failure has also been noted in patients with mesenchymal hamartoma due to arteriovenous shunting within the tumor. Patients can experience respiratory distress from the large, fluid-filled lesion if fluid accumulation has been rapid. Liver function tests are usually normal.
Sonographically, mesenchymal hamartoma is sometimes mistaken for hemangioma; however, although it frequently reveals internal septations demonstrating a complex appearance, it is avascular. These septations are strands of hepatocytes, bile duct elements, or mesenchyme separating multiple cysts. The hamartoma is usually situated in the right lobe.
The prognosis of mesenchymal hamartoma is excellent. Resection is usually all that is required, although in patients with respiratory distress, percutaneous drainage of the mass is performed before surgery.
The differential diagnosis of mesenchymal hamartoma includes mesenchymoma, hemangioma, parasitic or congenital cyst, teratoma, biliary cystadenoma, and choledochal cyst.
Rare benign lesions include focal nodular hyperplasia, hepatic adenoma, nodular regenerative hyperplasia, and fatty tumors; all of these tumors have the same clinical presentation, sonographic appearance, and complications in adults and children.
Cysts15
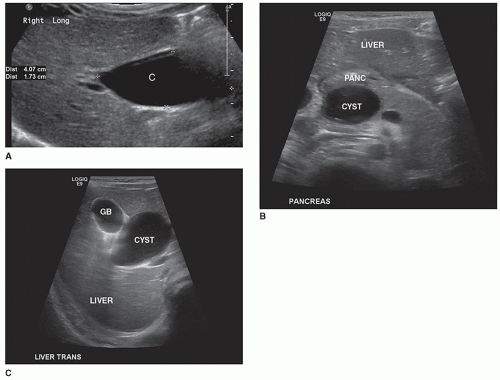
Congenital liver cysts are relatively rare. They range in size from small to large. Polycystic disease of the liver is seen with polycystic kidney disease and von Hippel-Lindau disease. Acquired cysts include hydatid cysts and traumatic cysts caused by blunt trauma. Hemobilia can be detected if there is communication with the biliary tree. The patient is generally asymptomatic unless the lesion is large enough to impair function and cause abdominal distention. The cyst may be palpated on physical examination or found incidentally on an imaging examination.
Sonographically, simple congenital liver cysts appear as smooth-walled, anechoic lesions demonstrating good posterior enhancement. They may be completely intrahepatic, partly intrahepatic, or completely extrahepatic and attached by a stalk.
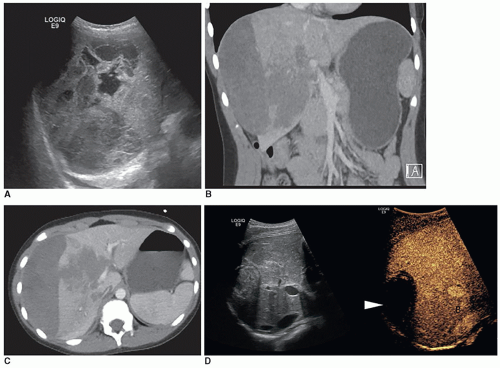
Hydatid echinococcal cysts or parasitic cysts are usually associated with exposure to livestock, farming, and dogs. After the eggs have been ingested, the gastric juices dissolve the covering of the embryo, allowing the organism to move spontaneously and attach itself to the intestinal wall. From there, it travels through the portal system to the liver, where it lodges and creates a cyst. The sonographic appearances include simple cyst, complex cysts (daughter cysts, echogenic septa, echogenic debris, or floating membranes), and simple or complex cysts with calcifications (Fig. 20-9A-D). The peak incidence occurs in patients aged 5 to 15 years. In this population, 25% are asymptomatic and 60% present with symptoms, including urticaria, right upper quadrant pain, and abdominal swelling due to hepatomegaly. As the lung is the second most common site affected, the right more often than the left, patients with pulmonary hydatid cyst present with pain on the affected side, coughing, high fever, and dyspnea. Forty percent develop complications including rupture into the peritoneal and pleural spaces, resulting in anaphylactic shock and pneumonia. Sometimes, the organism passes through the liver and lodges in the lungs, brain, kidneys, or elsewhere. Depending on the size and location of the lesions, the patient may experience infection, impaired liver function due to biliary obstruction, or other complications due to obstruction or compression of abdominal vasculature.
Treatment usually consists of aspiration, capitonnage, omentopexy, or a combination of two or more surgical procedures.
Differential diagnosis of extrahepatic cysts includes ovarian or mesenteric cysts, whereas the differential diagnosis
of an intrahepatic cyst includes teratoma, mesenchymoma, and tuberculin hepatic granuloma. It is important to distinguish an intrahepatic cyst from a choledochal cyst, which involves the bile duct and is discussed in “Gallbladder and Biliary System” section.
of an intrahepatic cyst includes teratoma, mesenchymoma, and tuberculin hepatic granuloma. It is important to distinguish an intrahepatic cyst from a choledochal cyst, which involves the bile duct and is discussed in “Gallbladder and Biliary System” section.
Hepatic Trauma15
The most commonly injured abdominal organ in blunt abdominal trauma in children is the liver, with the right lobe involved more often than the left lobe. The types of injuries to the liver include subcapsular and parenchymal hematomas, lacerations, and fractures. Hemoperitoneum is often noted in liver trauma injuries (Fig. 20-10A-D).
Hematomas of the liver demonstrate a change in echogenicity over time, progressing from anechoic, to complex, to anechoic with possible development of calcification. Gas or air secondary to tissue ischemia and necrosis may be noted. Biloma (walled-off collections of bile) and pseudoaneurysms may be later complications of liver trauma.
Infectious and Inflammatory Disease
Hepatitis16
Hepatitis is a diffuse infection of the liver characterized by inflammation and hepatic cell necrosis. Nearly all cases are viral in origin (hepatitis A, B, C, D, or E; cytomegalovirus, herpes, and Epstein-Barr). Noninfectious causes include toxin exposure, drugs, sclerosing cholangitis, and autoimmune disease. Type A is transmitted by a fecal-oral route of contaminated material. Children and young adults are most often infected by the hepatitis A virus. The extent of liver damage ranges from mild involvement to widespread necrosis and hepatic failure.
The clinical symptoms vary depending on the stage of the disease. The patient can experience abdominal swelling (hepatomegaly) with pain, nausea, fever, chills, jaundice, fatigue, or loss of appetite.
Depending on the stage of the disease, the sonographic appearance of the liver can range from hypoechoic to increasingly hyperechoic. In acute hepatitis, hepatomegaly
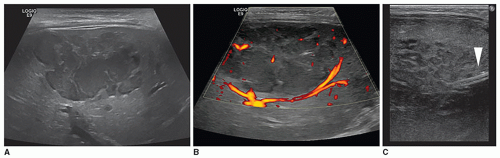
with decreased parenchymal echogenicity and increased echogenicity of the portal walls may be present. As the patient recovers, the size and echogenicity of the liver return to normal; however, with chronic hepatitis, the size of the liver may decrease but echogenicity and attenuation increase because normal liver tissue is destroyed and replaced by fibrosis and nodular regeneration. Chronic hepatitis may lead to cirrhosis, liver damage, and cancer. Therefore, routine screening sonogram is recommended in these patients. Sonography utilizing shear-wave elastography may be used to monitor the stiffness of the liver. Thickening of the gallbladder wall, small gallbladder filled with sludge, and enlarged nodes in the porta hepatitis can be found in cases of severe hepatitis (Fig. 20-11A-C).
with decreased parenchymal echogenicity and increased echogenicity of the portal walls may be present. As the patient recovers, the size and echogenicity of the liver return to normal; however, with chronic hepatitis, the size of the liver may decrease but echogenicity and attenuation increase because normal liver tissue is destroyed and replaced by fibrosis and nodular regeneration. Chronic hepatitis may lead to cirrhosis, liver damage, and cancer. Therefore, routine screening sonogram is recommended in these patients. Sonography utilizing shear-wave elastography may be used to monitor the stiffness of the liver. Thickening of the gallbladder wall, small gallbladder filled with sludge, and enlarged nodes in the porta hepatitis can be found in cases of severe hepatitis (Fig. 20-11A-C).
The etiology of abscess is related to the source of the infection and can be introduced to the liver by various routes, including trauma, direct invasion of adjacent structures, the hepatic artery, the portal vein, umbilical vein, or the bile ducts.
Laboratory values vary. The liver enzyme levels may be normal or elevated. Patients are not usually jaundiced. Blood cultures are generally negative. Leukocytosis is common but varies among patients. In neonatal abscesses, the organism is usually Gram negative rather than Gram positive.
Intrahepatic abscesses in infants present sonographically as in older patients and vary from discretely marginated hypoechoic structures with good sound transmission to complex hyperechoic masses with poorly defined margins. Lesions that contain gas (air) are hyperechoic with acoustic shadowing and reverberation artifacts. The mass may also present with a bull’s-eye appearance (a central hyperechoic area surrounded by a more anechoic one). In transplacental infection with calcifications, a bright, hyperechoic lesion with posterior shadowing can be seen (Fig. 20-12A-C).
Pyogenic liver abscess (PLA) is rare in children and can be fatal. Pyogenic abscess in children is secondary to generalized infections from the bowel (appendicitis or inflammatory
bowel disease), trauma, or surgery. Immunosuppression is an important predisposing condition. The most common causative agents are Escherichia coli and Klebsiella pneumoniae. PLA can also be seen in Crohn disease, chronic granulomatous disease, intestinal infection or bacteremia of any source, cholecystitis, biliary atresia, polycythemia, perforated viscus, Candida organisms, and hematopoietic malignancies.
bowel disease), trauma, or surgery. Immunosuppression is an important predisposing condition. The most common causative agents are Escherichia coli and Klebsiella pneumoniae. PLA can also be seen in Crohn disease, chronic granulomatous disease, intestinal infection or bacteremia of any source, cholecystitis, biliary atresia, polycythemia, perforated viscus, Candida organisms, and hematopoietic malignancies.
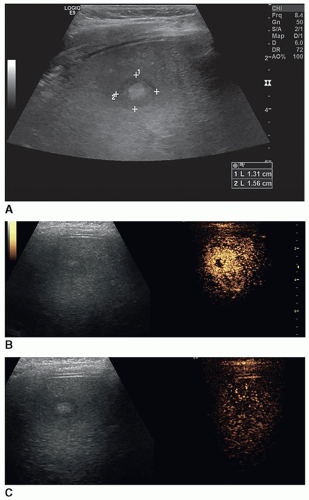
Fungal abscess occurs most often in the immunocompromised patient and is usually due to Candida albicans. This type of abscess is most commonly seen as multiple small lesions with irregular walls throughout the liver and may also be seen in the spleen and kidneys. Sonographically, the lesions can appear round and hypoechoic, or hyperechoic, or have a target or wheel-within-wheel appearance (Fig. 20-13).
Amebic liver abscess, although an adult disease, also affects children in areas, where drinking water is contaminated and sanitation is poor. Hepatic abscess is the main complication of the organism Entamoeba histolytica, forming in 1% of the population who is infected.
E. histolytica enters the liver from the colon via the portal system and forms a cavity that becomes the abscess. The organism resides in the wall of the abscess, and the right lobe is more commonly affected. Sonographically, the abscess can be readily identified as a hypoechoic, spherical lesion. After treatment, it can be followed with serial sonography.
Diffuse Liver Disease
Diffuse parenchymal diseases include fatty infiltration, hepatic fibrosis, cirrhosis, hemosiderosis, and metabolic diseases.
Fatty infiltration of the liver is caused by chronic hepatic injury and results from an accumulation of abnormal amounts of triglycerides and lipids in the hepatocytes. Fatty infiltration may be diffuse or focal, and in children, it can be related to a variety of conditions, including malignancies, metabolic diseases, cystic fibrosis, and exposure to liver toxins. However, as childhood obesity has emerged as a significant health problem worldwide, the prevalence of fatty infiltration of the liver has increased in children. Fatty infiltration is being seen at a younger age and without the presence of other underlying risk factors.
Diffuse fatty infiltration results in hepatomegaly and sonographic findings of increased parenchymal echogenicity and attenuation of the sound beam. The resulting sonographic appearance is similar to that of older patients, including a large echogenic liver with decreased visualization of the intrahepatic vessels, posterior portions of the liver, and the diaphragm. Less common patterns of fat deposition include focal, multifocal, perivascular, and subcapsular deposition.
Metabolic Liver Disease
Metabolic liver disease is a group of disorders affecting the liver. Inborn errors of metabolism are usually due to a defect in an enzyme or transport protein that causes abnormalities in the synthesis or catabolism of proteins, carbohydrates, or fats. This is not the same as metabolic disease (or syndrome) in the adult.
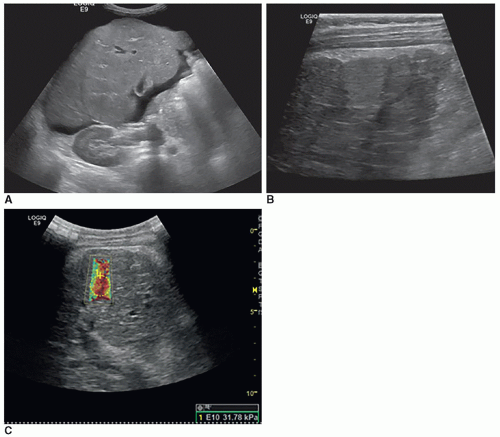
These diseases may directly damage the liver, resulting in cirrhosis or liver failure. Or they may be due to a metabolic defect in the liver, causing damage to other organ systems. Metabolic disorders of the liver include glycogen storage disease (type I von Gierke disease is the most common), lipodystrophy, cystic fibrosis, Gaucher disease, and Wilson disease, all of which have the sonographic appearance of fatty infiltration of the liver (Fig. 20-14A-C).
Cirrhosis is parenchymal destruction, scarring, fibrosis, and nodular regeneration of the liver (Fig. 20-15A-C). In infants and children, it is due to biliary atresia, cystic fibrosis, chronic hepatitis, metabolic disease (Wilson disease, glycogen storage disease, tyrosinemia, galactosemia, and
alpha1-antitrypsin deficiency), prolonged parenteral nutrition, Budd-Chiari syndrome, and medications. The clinical, laboratory, and sonographic presentation is the same as in adults. Secondary signs of ascites, splenomegaly, and portal hypertension may be present.
alpha1-antitrypsin deficiency), prolonged parenteral nutrition, Budd-Chiari syndrome, and medications. The clinical, laboratory, and sonographic presentation is the same as in adults. Secondary signs of ascites, splenomegaly, and portal hypertension may be present.
Hepatic Fibrosis23
Hepatic fibrosis occurs in the absence of cirrhosis and has been associated with metabolic disorders, cystic fibrosis, biliary atresia, liver transplantation, severe congenital heart disease, cardiac transplantation, and autosomal recessive polycystic disease. Hepatomegaly and portal hypertension are common symptoms. Sonographically, the liver demonstrates increased echogenicity and biliary dilatation because of the presence of dense fibrous bands surrounding the liver lobules.
Increased echogenicity of the kidneys may also be noted.
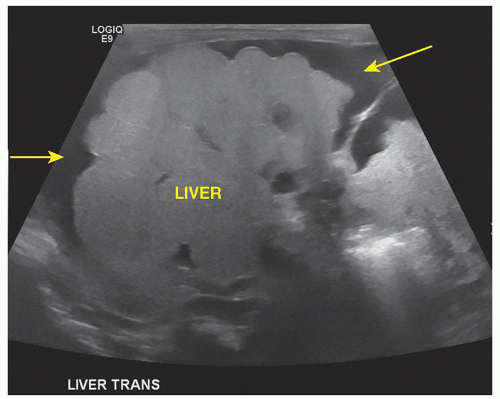
Hemochromatosis occurs when an excessive amount of iron is stored within the liver. Hemochromatosis may be genetic, secondary, or transfusional. Hemosiderosis is iron storage in the liver resulting from repeated blood transfusions. The liver may demonstrate a decrease in echogenicity (Fig. 20-16). Magnetic resonance imaging (MRI) is the best imaging test for detecting hemosiderosis.
Primary malignant tumors of the liver are more common in children than in adults, and two-thirds of all pediatric hepatic tumors are malignant. These tumors include hepatoblastoma, HCC (hepatoma), mesenchymal (embryonal) sarcoma, and congenital neuroblastoma. Other rare liver tumors include rhabdomyosarcoma, angiosarcoma, germ cell tumors, and undifferentiated sarcomas. Primary liver tumors account for 2% to 5% of all malignant pediatric tumors. The AFP level is usually elevated in the presence of malignant hepatic tumors, and invasion of surrounding vessels is commonly noted. Because vascular invasion can impact the treatment decisions of hepatic malignancies, sonography is of vital importance to identify extension of the tumor into major blood vessels and differentiate this from thrombus formation.
Sonographically, malignancies usually demonstrate as a solitary, solid, homogeneous, hyperechoic mass and less frequently as multiple hyperechoic lesions. In some cases, a hypoechoic halo or rim may be seen, and infrequently, the malignancy may be isoechoic to normal liver tissue.
Hepatoblastoma is the most common pediatric liver mass, occurring most commonly in boys younger than 5 years of age.
Hepatoblastoma is associated with Beckwith-Wiedemann syndrome (hemihypertrophy, macroglossia, hypoglycemia, organomegaly, and omphalocele), fetal alcohol syndrome, development of Wilms tumor, dysplastic kidney, and Meckel diverticulum.
Hepatoblastoma is associated with Beckwith-Wiedemann syndrome (hemihypertrophy, macroglossia, hypoglycemia, organomegaly, and omphalocele), fetal alcohol syndrome, development of Wilms tumor, dysplastic kidney, and Meckel diverticulum.
A tumor should be considered resectable if it does not occupy more than one lobe, has no extrahepatic extension, and does not invade the portal vein. Although hepatoblastomas are often detected in advanced stages, unresectable tumors can be biopsied and converted to resectable tumors by chemotherapy. Chemotherapy is applied before surgery to shrink the tumor, resulting in improved operability.
Clinically, patients usually present with hepatomegaly or a painless, palpable abdominal mass in 90% of cases. In advanced cases, there can be accompanying fever, weight loss, pain, nausea, vomiting, jaundice, anemia, leukocytosis, adenopathy, and fractures due to bone metastases. Laboratory values include an elevation of AFP in 84% to 91% of cases, with a decrease after resection. There may also be a transaminase elevation, as well as anemia and thrombocytosis.
Sonographically, a hepatoblastoma appears as a solitary multinodular mass with a heterogeneous, hyperechoic pattern and indistinct borders (Fig. 20-17A-C).
Anechoic foci may also be present, representing necrosis or hemorrhage. Dense or coarse calcifications with posterior shadowing are also common. The differential diagnosis includes HCC, infantile hemangioendothelioma, and mesenchymal hamartoma.
HCC, which is also known as hepatoma, affects children older than 3 years of age and has been associated with chronic
liver diseases, such as type I glycogen storage disease, Wilson disease, biliary atresia, and hepatitis. Pathologically, these lesions have characteristics that differentiate them from other hepatic lesions: daughter nodules, hepatic or portal tumor thrombosis, septa, and pseudocapsules. This tumor can be either well encapsulated or nonencapsulated and is commonly multicentric.
liver diseases, such as type I glycogen storage disease, Wilson disease, biliary atresia, and hepatitis. Pathologically, these lesions have characteristics that differentiate them from other hepatic lesions: daughter nodules, hepatic or portal tumor thrombosis, septa, and pseudocapsules. This tumor can be either well encapsulated or nonencapsulated and is commonly multicentric.
Clinically, the patient presents with sudden liver failure due to invasion of the tumor or thrombosis in the portal or hepatic veins, hepatomegaly, pain, GI bleeding, ascites, anorexia, hypoglycemia, anemia, weakness, and fever. Laboratory values include elevated AFP in 60% to 80% of cases.
Sonographically, the tumor may appear similar to a hepatoblastoma. It generally presents as a solid, hyperechoic mass and usually involves the entire liver. It can have well-defined or ill-defined borders. There may be anechoic areas within the mass representing necrosis or hemorrhage. An anechoic or hypoechoic halo or rim may also be seen. Tumor thrombi are frequently seen in the portal veins, hepatic veins, and IVC and should be documented if present.
The outcome for cirrhotic patients who develop HCC is poor. The differential diagnosis includes hepatoblastoma, abscess, focal nodular hyperplasia, adenoma, hemangiosarcoma, hemangioendothelioma, and biliary rhabdomyosarcoma.
Fibrolamellar Hepatocellular Carcinoma10
Fibrolamellar HCC is histologic subtype of HCC, which most commonly affects teenagers and young adults. Clinical findings include abdominal pain, mass, fever, weight loss, diarrhea, and vomiting. AFP levels are typically normal or mildly elevated. The tumor is usually solitary and well marginated with variable echogenicity. Some tumors demonstrate a central scar and/or focal calcifications. The sonographic appearance is so similar to other solid hepatic neoplasms that biopsy is needed to differentiate these tumors.
Mesenchymal (embryonal) sarcoma is a rare malignant liver tumor that typically presents in patients aged 5 to 10 years, as a large fast-growing, round, singular mass with well-defined borders and a thick, fibrous pseudocapsule usually within the right lobe. It can contain multiple cystic spaces, hemorrhage, necrosis, and brown viscous material, as well as fibrous bands. It can easily spread to the abdominal cavity or to the diaphragm, with metastases to the lungs. Mesenchymal sarcoma is the fourth most common primary pediatric liver tumor following hepatoblastoma, infantile hemangioendothelioma, and HCC. Subtypes of mesenchymal
sarcoma include embryonal sarcoma, rhabdomyosarcoma, angiosarcoma, and malignant mesenchymoma.
sarcoma include embryonal sarcoma, rhabdomyosarcoma, angiosarcoma, and malignant mesenchymoma.
The clinical findings include abdominal pain and swelling, with a palpable mass. Jaundice is usually not present, and the AFP level is not increased.
Sonographically, mesenchymal sarcoma usually presents as a single hyperechoic mass containing anechoic areas, which represent the cystic spaces. It can also appear homogeneous and hyperechoic or as a complex lesion with anechoic areas as well as calcifications producing posterior shadowing.
Metastases10
The most common cause of metastatic hepatic neoplasms in children is neuroblastoma. Hepatic metastases are frequently associated with Wilms tumor, neuroblastoma, leukemia, and lymphoma. As in adults, the echogenicity and echotexture of metastases is variable: hypoechoic, isoechoic, hyperechoic and homogeneous, and heterogeneous or complex. Calcifications may be noted. Rarely are pediatric metastases diffuse, with diffuse disease most commonly associated with stage IV-S neuroblastoma.
Lymphoproliferative disorder can be a complication of solid organ transplant. Single or multiple hypoechoic masses in the liver may be noted sonographically; occasionally, diffusely infiltrating disease is found (Fig. 20-18A, B).
Lymphoma of the liver is more commonly secondary to non-Hodgkin lymphoma. Sonographically, discrete hypoechoic nodules are noted; hepatosplenomegaly may be present.
Hepatic Vascular Disorders
Vascular disorders of the liver include portal hypertension, Budd-Chiari syndrome, portal vein thrombosis, hepatic infarction, peliosis hepatis, and portal venous gas.
Portal hypertension is due to increased resistance to normal portal venous flow. The clinical presentation includes splenomegaly, ascites, caput medusa, and, in severe cases, hematemesis, hepatic encephalopathy, and hypersplenism. The obstruction to flow can be prehepatic (portal or splenic vein thrombosis), intrahepatic (secondary to cirrhosis and, less commonly, hepatic vein obstruction), or posthepatic (secondary to congestive heart failure or constrictive pericarditis).
Sonographic findings include bidirectional or hepatofugal portal vein flow, development of varices, splenomegaly, a thicken lesser omentum, ascites, and evidence of cirrhosis. The portal vein may be dilated, but the size and number of varices or collaterals that develop as the disease progresses can reduce the diameter of the main portal vein. Respiratory variation in portal venous flow may be absent or reduced. Flow in the hepatic artery may increase to compensate for the decrease in blood to the liver via the main portal vein. The hepatic veins may demonstrate a loss of pulsatility and a monophasic flow pattern.
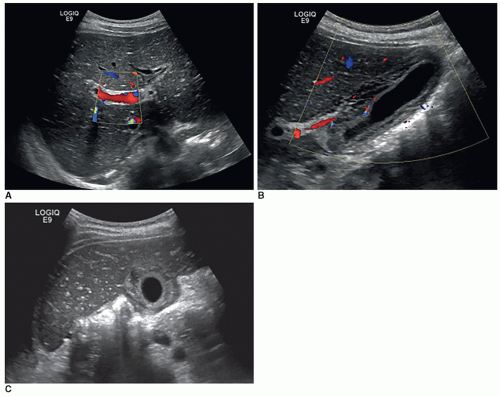
Portal vein thrombosis can be caused by thrombus or tumor invasion. Hepatoblastoma and HCC may involve tumor invasion of the portal vein. Nonmalignant thrombosis is associated with improper placement of UVC, dehydration, shock, sepsis, hypercoagulable states, and portal hypertension. Clinical presentation includes acute abdominal pain and, in some cases, splenomegaly. Portal vein thrombosis can be acute (enlarged, echogenic vein, absent flow with color Doppler or, in cases of tumor invasion, flow within the thrombus) or chronic (cavernous transformation of the portal vein, which is described as multiple tortuous vessels in the porta hepatis and nonvisualization of the portal vein).
Acute portal vein thrombosis may be anechoic and mimic a patent portal vein on grayscale imaging; however, color Doppler will confirm the finding of thrombosis. Additional sonographic findings in chronic portal vein thrombosis include the development of collaterals with the addition of pericholecystic collaterals in some cases (Fig. 20-19A-D).
Budd-Chiari syndrome may be due to idiopathic occlusion or neoplastic invasion of the hepatic veins, usually secondary to hepatoblastoma, HCC, Wilms tumor, or thrombosis. Idiopathic causes of occlusion include hypercoagulable states, trauma, Gaucher disease, and cirrhosis. The primary sonographic findings include hepatomegaly, echogenic
intraluminal clot, and the absence of hepatic vein flow using color and pulse-wave Doppler (Fig. 20-20A, B).
intraluminal clot, and the absence of hepatic vein flow using color and pulse-wave Doppler (Fig. 20-20A, B).
Secondary findings include ascites, pleural effusion, and gallbladder wall edema (Fig. 20-20C).
Nonvisualization of the hepatic veins is not specific evidence of hepatic vein occlusion because patent veins can be difficult to identify in the presence of hepatomegaly or cirrhosis. In the chronic stages, additional collateral pathways for hepatic vein flow can develop.
Occlusion of the vena cava may be due to a congenital membrane within the IVC (noted sonographically as a thin, hyperechoic band inside the IVC), neoplastic invasion, extrinsic tumor compression, enlarged caudate lobe, and thrombosis. Obstruction of the IVC can cause hepatic venous congestion and development of thrombus in the hepatic veins. Color and pulse-wave Doppler confirm the absence of flow in the obstructed portion of the IVC and abnormal flow in the hepatic veins. The IVC may be dilated inferior to the obstruction.
Portal Venous Gas
Air in the portal vein and its branches can be associated with umbilical venous catheterization, bowel surgery, and neonatal gastroenteritis. However, it is particularly important to recognize this entity in neonates because it can result from mesenteric ischemia due to small bowel obstruction or NEC, which occurs predominantly in premature and low birth weight infants with significant morbidity and mortality. Multiple echogenic foci can be seen moving within the vessels in the direction of blood flow, but acoustic shadowing and reverberation are not usually noted (Fig. 20-21A, B).
GALLBLADDER AND BILIARY SYSTEM29
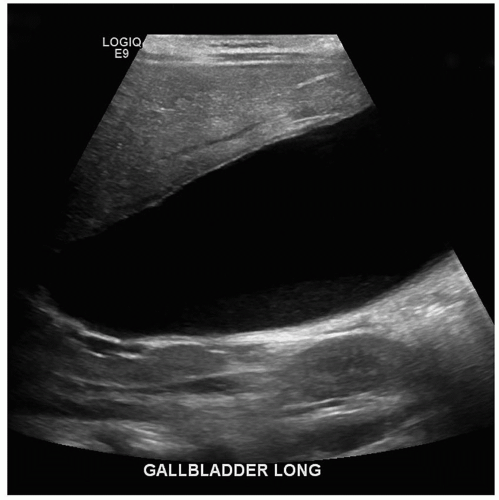
Although gallbladder disease is uncommon in children, it does occur. The normal sonographic appearance of the gallbladder is the same as in the adult patient: thin-walled, well-defined structure, with an anechoic lumen and echogenic walls.
Sonographic Examination Technique
Scan Technique
Patients are most commonly scanned in the supine and LPO positions. Additional patient positions, such as prone (especially in obese children), semi-erect, erect, and right posterior oblique (RPO), may be helpful.
The sonographer should acquire documentary images that clearly demonstrate the gallbladder and bile ducts in longitudinal and transverse planes. Evaluation, assessment, and documentation should include the gallbladder periphery; longitudinal and transverse sweeps extending past the medial, lateral, superior, and inferior borders of the gallbladder; and the total length of the bile duct should be evaluated. Normal maximal diameter of the common bile duct is calculated by age in children.
Congenital anomalies of the biliary tract include biliary atresia, choledochal cyst, and, rarely, gallbladder ectopia, agenesis, or duplication (Fig. 20-22).
Elevated lab values of conjugated bilirubin (sometimes referred to as direct bilirubin) in the newborn have two major causes: diseases of the liver such as hepatitis and biliary tract abnormalities such as atresia. Signs and symptoms of neonatal cholestasis and neonatal hepatitis are similar to those of biliary atresia. In these conditions, patients present with jaundice at about 3 to 4 weeks of age. When infectious causes have been excluded, biliary atresia should be suspected.
It is extremely important to determine whether biliary atresia is present, because early identification significantly improves the clinical outcome of the patient. Biliary atresia requires surgical intervention, whereas neonatal hepatitis is treated medically. There are two surgical interventions for biliary atresia. Initially, the Kasai procedure is performed to develop a communication between the liver and the duodenum to promote drainage of bile and prevent liver failure. The success rate of this procedure is greatest when the intervention is performed before 8 weeks of age. The other treatment for biliary atresia is liver transplantation.
It is important to make sure that the patient is fasting appropriately to ensure visualization of the normally distended gallbladder. The manifestations of biliary atresia range from total absence of the biliary tree to a visibly patent gallbladder, cystic duct, and common bile duct. Sonographically,
the gallbladder, cystic duct, common bile ducts, and intrahepatic bile ducts may or may not be seen, depending on the degree of atresia. Most commonly, the intrahepatic and extrahepatic bile ducts near the porta hepatis are absent. If a small, atretic gallbladder is seen, it can be reevaluated with extended fasting (up to 5 hours) to confidently demonstrate a lack of normal distention. If a rudimentary gallbladder is seen, a fasting measurement of less than 1.5 cm suggests atresia. It can also be checked postprandial to see if the size has changed. If it is not connected to the biliary system, there should be no change in its size.
the gallbladder, cystic duct, common bile ducts, and intrahepatic bile ducts may or may not be seen, depending on the degree of atresia. Most commonly, the intrahepatic and extrahepatic bile ducts near the porta hepatis are absent. If a small, atretic gallbladder is seen, it can be reevaluated with extended fasting (up to 5 hours) to confidently demonstrate a lack of normal distention. If a rudimentary gallbladder is seen, a fasting measurement of less than 1.5 cm suggests atresia. It can also be checked postprandial to see if the size has changed. If it is not connected to the biliary system, there should be no change in its size.
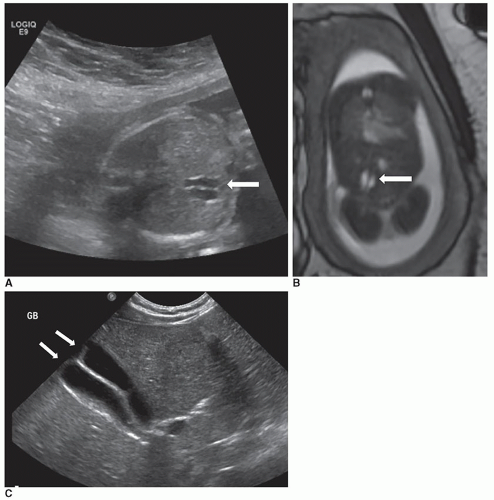
The triangular cord sign is an important sonographic finding in biliary atresia and is seen as an echogenic tubular focus near the anterior branch of the right portal vein measuring greater than 4 mm in thickness. The liver is enlarged and diffusely hyperechoic. Microcyst in the porta hepatis may be noted. An increased hepatic artery diameter (>2 mm), splenomegaly (>6 mm), and polysplenia are also associated with biliary atresia (Fig. 20-23 A-C). Ascites may be present. Shear-wave elastography shows promise as a method for differentiating biliary atresia from other hepatic pathologies.
Some patients with biliary atresia also have other congenital anomalies, such as anomalous origin of the hepatic artery, azygous continuation of the IVC, bilaterally bilobed lungs, preduodenal portal veins, abdominal malrotation, and visceral situs anomalies.
Care must be taken to ensure that a choledochal cyst is not mistaken for a normal gallbladder. The absence of the gallbladder may be the only sonographic sign of biliary atresia.
Choledochal cyst is a congenital dilatation of the common bile duct that presents as abdominal pain, mass, and jaundice. Both sonography and hepatobiliary scintigraphy are used to establish the diagnosis of this disease, with CT and MRI providing additional information.
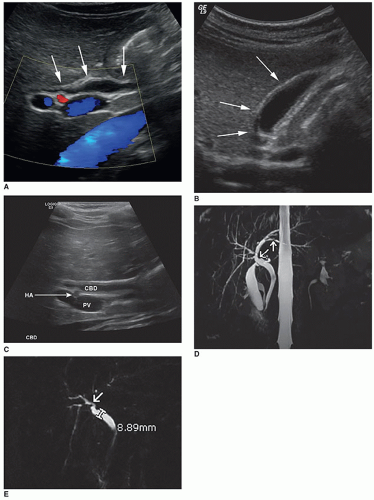
There are five main types of choledochal cyst. Type I is the fusiform dilatation of the common bile duct and is the most common form found in infants and children (Fig. 20-24A, B).
Type II presents as a diverticulum of the common bile duct and is the second most common (Fig. 20-25A).
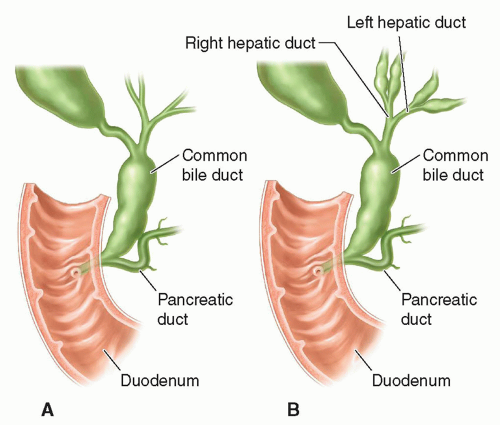 FIGURE 20-24 Choledochal cyst. A: Type I: concentric dilatation. B: Type IV: fusiform dilatation, concentric dilatation, with intrahepatic involvement. |
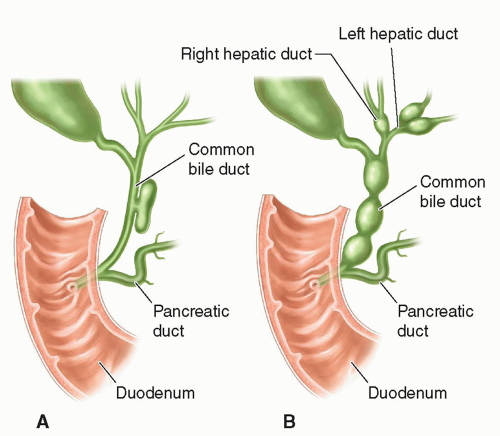 FIGURE 20-25 Choledochal cyst. A: Type II: eccentric common bile duct diverticulum. B: Type IV: rosary common bile duct diverticulum. |
Type III is a congenital choledochocele, which is a cystic dilatation of the intraduodenal portion of the common bile duct. Type IV choledochal cysts are concentric dilatations of the common bile duct with intrahepatic ductal dilatation (Figs. 20-24B and 20-25B). Type V is Caroli disease in which the peripheral intrahepatic ducts are affected either diffusely or focally (Fig. 20-26).
Sonographically, a type I choledochal cyst appears as fluid-filled, well-defined mass in the porta hepatis adjacent to the gallbladder (Fig. 20-27A-C). The right, left, and common bile ducts may be seen entering the cyst, and the gallbladder is demonstrated as a separate cystic structure. If the cyst is large, it may contain sludge. A type II choledochal cyst demonstrates one or more diverticula or fluid-filled structures near or coming off the common bile duct. If there is intrahepatic ductal dilatation, type IV should be considered, and if peripheral ductal dilatation is identified, Caroli disease (type V) cannot be ruled out. In types III and IV, intrahepatic ductal dilatation is noted.
Biliary atresia may be concurrent, in which case the choledochal cyst will be smaller and the intrahepatic ductal dilatation is absent.
Complications of untreated choledochal cyst include stone formation within the cyst, gallbladder or pancreatic duct, chronic biliary obstruction, chronic cholangitis, cirrhosis, biliary rupture with resulting biliary peritonitis, neoplasia (risk of adenocarcinoma increases with age), and pancreatitis.
Abnormal Gallbladder Size
If the patient is nonfasting, the gallbladder should be contracted (nondistended). In a fasting patient (4 to 6 hours), a small or nondistended gallbladder may indicate biliary atresia, congenital hypoplasia, acute viral hepatitis (AVH), cystic fibrosis, or chronic cholecystitis (uncommon in children). A large gallbladder may indicate prolonged fasting, hydrops, or obstruction of the cystic or common bile ducts. Administration of a fatty meal can be used in cases of gallbladder enlargement to determine whether the cystic duct is patent. The gallbladder is scanned before the fatty meal and should show emptying 45 minutes to 1 hour after the fatty meal if the cystic duct is patent.
Nonvisualization of the gallbladder is most commonly associated with biliary atresia or viral hepatitis. Less common etiologies include agenesis, ectopia, normal contraction after a meal, and the presence of sludge. A sludge-filled gallbladder can be isoechoic with the liver, making sonographic detection difficult.
Diffuse gallbladder wall thickening is a nonspecific finding associated with numerous inflammatory and noninflammatory causes. In children, as in adults, a gallbladder wall thickness of 2 to 5 mm suggests disease, and thickness of 5 mm or more is considered indicative of disease. Inflammatory causes include acute and chronic cholecystitis. Noninflammatory causes include viral hepatitis, hepatic dysfunction, cirrhosis, hypoalbuminemia, pancreatitis, congestive heart
failure, renal disease, bone marrow transplant, sepsis, and AIDS. Diffuse wall thickening may have several sonographic appearances, including uniformly echogenic, hypoechoic, or striated (hypoechoic and hyperechoic layers). In patients who are nonfasting, the gallbladder wall will demonstrate thickening because of the lack of distention of the gallbladder. Focal wall thickening is associated with cholecystitis or adenomyomatosis.
failure, renal disease, bone marrow transplant, sepsis, and AIDS. Diffuse wall thickening may have several sonographic appearances, including uniformly echogenic, hypoechoic, or striated (hypoechoic and hyperechoic layers). In patients who are nonfasting, the gallbladder wall will demonstrate thickening because of the lack of distention of the gallbladder. Focal wall thickening is associated with cholecystitis or adenomyomatosis.
Cholelithiasis is the presence of one or more calculi (stones) in the gallbladder, cystic duct, or common bile duct. Biliary obstruction occurs if calculi are situated in the cystic or common bile ducts. Because bile salt secretion in infants is 50% of that in adults, it is assumed that any treatment that suppresses bile acid formation greatly increases the risk of gallstones. The incidence of gallstones is rising in children owing to the increase in childhood obesity, and pigmented stones are more common in children than cholesterol stones. Children with sickle cell disease have an increased incidence of cholelithiasis that is nearly double the general population.
Neonatal cholelithiasis is associated with congenital anomalies of the biliary system, total parenteral nutrition (TPN), dehydration, infection, hemolytic anemia, extracorporeal membranous oxygenation (ECMO), and short-gut syndrome. Common causes of gallstones in older children and teenagers include cystic fibrosis, malabsorption, TPN, liver disease, Crohn disease, bowel resection, sickle cell disease, medication use by pediatric patients for congenital heart disease, and hemolytic anemia. Most children with gallstones are predisposed because of the presence of an underlying disease process; however, some gallstones are idiopathic.
The clinical presentation in younger children with gallstones includes nonspecific symptoms (jaundice, irritability), whereas older children and teens present with more classic symptoms of right upper quadrant pain, intolerance to fatty foods, nausea, and vomiting. The most common complication of gallstones in children is pancreatitis. Often, the gallstones resolve without treatment.
Gallstones and a sludge-filled gallbladder present sonographically as in older patients.
A neonate may be examined because gallstones were noted on a fetal sonogram; most resolve spontaneously within the first year of life. The formation of sludge within the gallbladder is associated with prolonged fasting, hyperalimentation, and extrahepatic bile duct obstruction. Patients with sickle cell disease and cystic fibrosis are predisposed to the formation of sludge.
Cystic duct stones are very difficult to demonstrate if the duct is not dilated. An impacted cystic duct stone can compress the adjacent common bile duct (Mirizzi syndrome), causing extrinsic bile duct dilatation and obstructive jaundice. Common bile duct stones can result in further complications, such as biliary obstruction, cholangitis, or pancreatitis.
Acute or chronic cholecystitis in children has a number of causes: hypoalbuminemia, AVH, heart failure, renal failure, gallbladder carcinoma, ascites, multiple myeloma, congenital obstruction of the cystic duct or obstruction from an external source, biliary stasis, and portal node lymphatic obstruction.
Clinically, the patient can present with right upper quadrant pain, fever, vomiting, and a palpable right upper quadrant lump. The differential diagnosis includes cholecystitis, abdominal abscesses of the right upper quadrant, pancreatitis, appendicitis, peptic ulcer disease, and gallbladder torsion.
In a diseased state, the gallbladder wall presents sonographically as thickened, irregular, and highly reflective. A hypoechoic to anechoic halo seen surrounding the gallbladder wall is usually due to either infection of the wall itself or a disease process in the surrounding liver tissue (Fig. 20-28A, B). At times, sludge can be seen in the gallbladder owing to stasis.
Hydropic Gallbladder42
Hydropic gallbladder develops in acutely ill children who receive TPN or hyperalimentation therapy and in association with group B streptococcal sepsis, congestive heart failure, shock, chronic biliary tract obstruction, upper respiratory tract infection, gastroenteritis, and Epstein-Barr virus infection. Diseases such as Kawasaki (mucocutaneous lymph node) syndrome, leptospirosis, typhoid fever, ascariasis, Salmonella, or Pseudomonas may also present with gallbladder hydrops. Clinically, patients present with right upper quadrant pain, fever, dehydration, and abdominal distention. The reasons for hydropic gallbladder are unclear. Most of the time, a hydropic gallbladder resolves spontaneously.
Sonographically, the gallbladder is dramatically enlarged and completely anechoic with thin walls. Gallstones or sludge may be present. Such a gallbladder generally does not contract well, following a fatty meal (Fig. 20-29).
Intrahepatic and extrahepatic bile duct obstruction may be due to the presence of neoplasm (rhabdomyosarcoma is the most common), enlarged lymph nodes in the porta hepatis compressing the bile duct, acute pancreatitis, biliary calculi, and biliary stricture (uncommon). Patients with biliary obstruction present with jaundice. Sonographically, the dilated intrahepatic bile ducts demonstrate as multiple anechoic irregularly branching structures, which are larger at the porta hepatis. The extrahepatic bile ducts demonstrate as round
or tubular anechoic structures near the porta hepatis and/or head of the pancreas. The sonographer should search for the point of obstruction and demonstrate the presence of a mass or calculus that is causing the obstruction. Pancreatitis usually causes the duct to taper significantly at the pancreatic head, whereas a mass, calculus, or stricture demonstrates as an abrupt change from a dilated to a narrowed or absent duct.
or tubular anechoic structures near the porta hepatis and/or head of the pancreas. The sonographer should search for the point of obstruction and demonstrate the presence of a mass or calculus that is causing the obstruction. Pancreatitis usually causes the duct to taper significantly at the pancreatic head, whereas a mass, calculus, or stricture demonstrates as an abrupt change from a dilated to a narrowed or absent duct.
Dilated bile ducts may spontaneously rupture, causing neonatal jaundice and bile ascites or a biloma. The most common location for perforation is at the junction of the cystic and common bile ducts. Affected patients usually present in the first 3 months of life with ascites, mild jaundice, failure to thrive, and abdominal distention. Bilirubin values are elevated, but all of the other liver function tests are normal; this distinguishes biliary obstruction from neonatal hepatis syndrome and biliary atresia.
Bile plug syndrome (inspissated bile syndrome) can cause obstruction of the bile ducts. Liver abnormalities are not present in this syndrome, which primarily affects full-term infants. Risk factors include massive hemolysis, TPN, Hirschsprung disease, cystic fibrosis, and intestinal atresias. Echogenic material may be found within dilated ducts, and sludge may be noted in the gallbladder. Bleeding into the ducts from trauma, surgery, or biopsy can mimic the sonographic appearance of bile plug syndrome.
Sclerosing cholangitis is a chronic disease in which there is inflammatory fibrosis that obliterates the intrahepatic and extrahepatic bile ducts. This leads to the development of biliary cirrhosis, portal hypertension, and liver failure. Approximately 70% to 80% of children with sclerosing cholangitis have concurrent inflammatory bowel disease. The clinical presentation of sclerosing cholangitis is right upper quadrant pain and jaundice with abnormal liver function tests and elevated bilirubin.
Sonographic findings include thickening of the walls of the bile ducts, choledocholithiasis (intrahepatic and extrahepatic), cholelithiasis, and ductal strictures (Fig. 20-30A-E).
Biliary Neoplasm
Biliary rhabdomyosarcoma is a rare soft-tissue tumor occurring in children, usually between the ages of 1 and 5 years. It is the second most common cause of obstructive jaundice in older children after choledochal cyst and in neonates after biliary atresia.
Clinical signs are increasing abdominal girth, vomiting, pain, and weight loss. Usually, the diagnosis is delayed because these clinical symptoms are confused with those of infectious hepatitis. Laboratory values may include elevated total serum bilirubin, a marked increase in alkaline phosphatase, and normal or mildly elevated aspartate transaminase (AST). There may also be a moderate increase in the white blood cell count owing to subsequent cholangitis. Unlike hepatoblastoma or HCC, biliary rhabdomyosarcoma may not cause an increase in the AFP level. Biopsy and histologic examination are definitive for diagnosis.
Sonographically, rhabdomyosarcoma is predominantly solid, with hyperechoic formations and no posterior shadowing, as is commonly seen with stones. The tumor appears lobulated and is usually situated in the hilum of the liver. There may be cystic spaces representing intrahepatic radicles of the bile ducts. There may also be focal areas of necrosis and hemorrhage in the mass. Usually, dilated bile ducts surround the mass. Frequently, it is misdiagnosed as a choledochal cyst.
The favored method of treatment is surgery with adjuvant chemotherapy and radiation therapy.
PANCREAS
Sonography is currently the diagnostic procedure of choice for the examination of children with symptoms of pancreatic disease. Real-time sonography of the pancreas in infants and children is easily performed. Compared with adults, the pancreas is more easily seen in children because most are lean and have a large left hepatic lobe, which serves as an excellent window for visualizing the pancreas. The drawbacks of pancreatic sonography include technically unsatisfactory studies due to obesity or excessive bowel gas, and limited scanning surfaces when surgical dressings or ostomy sites are present.
Sonographic Examination Technique
Scan Technique
A standard examination of the pancreas includes transverse and longitudinal scans of the supine patient. The transverse scans may require some initial survey to determine the exact position of the gland because it generally lies oblique in the middle portion of the body, with the head lower than the body and tail. Longitudinal scans should be oriented to the true longitudinal axis of the pancreas, as determined by the transverse scans. It is common to examine the patient in different positions (i.e., upright, decubitus) to adequately visualize the pancreas. This is particularly important in the presence of disease because the scan must demonstrate the lesion’s relationship to surrounding pancreatic structure and adjacent organs. Much of the pancreatic body and tail can be visualized with a coronal approach using the spleen as a sonographic window. Another helpful technique is to use the water-filled duodenum to outline the pancreas. The patient is given approximately 16 oz of water, and the progress of the water into the duodenum is checked periodically by the sonographer. When the duodenum is appropriately distended, the patient is repositioned until the water-filled duodenum outlines the area of the pancreas that is of interest. A fluid-filled stomach may also be helpful to outline the pancreatic tail. This technique, however, has several drawbacks: (1) many patients suffer severe nausea, and large amounts of water may induce vomiting; (2) fluid filling is contraindicated for fasting patients receiving intravenous fluid; and (3) the method can be time-consuming.
Technical Considerations
During pancreatic sonography, the gain control is usually at settings comparable to those used for scanning the liver. Determination of the normal pancreatic sonographic pattern
is based on a comparison to the liver parenchymal pattern. In children, the normal pancreatic parenchyma is relatively homogeneous, with even, high-, and medium-level echo distribution. This is in contrast to the irregular echo texture, or “cobblestone” appearance, considered normal for adults. The normal pancreas is similar to or more echogenic than the liver parenchyma. Vascular structures abound in this area and should have a clearly echo-free pattern and not filled in by too high a gain setting. The transducer frequency selections should be made to obtain the highest resolution with adequate penetration.
is based on a comparison to the liver parenchymal pattern. In children, the normal pancreatic parenchyma is relatively homogeneous, with even, high-, and medium-level echo distribution. This is in contrast to the irregular echo texture, or “cobblestone” appearance, considered normal for adults. The normal pancreas is similar to or more echogenic than the liver parenchyma. Vascular structures abound in this area and should have a clearly echo-free pattern and not filled in by too high a gain setting. The transducer frequency selections should be made to obtain the highest resolution with adequate penetration.
Normal Anatomy
During a sonographic pancreatic study, the sonographer should pay particular attention to surrounding vascular landmarks, to the gland’s shape and size, and to delineation of the pancreatic duct.
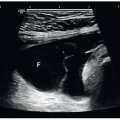
Attention to the surrounding vascular landmarks may be necessary to identify this rather small structure. Sonographic studies should also give specific attention to the size of the pancreas. The maximum anteroposterior (AP) diameters of the head, body, and tail are measured on transverse images obtained by angling the transducer to visualize more parenchyma. The entire gland can usually be seen in one image if it is oriented transversely across the abdomen, but often it lies oblique to some degree, with the tail more cephalad than the head and body, in which case it may be necessary to obtain several images to demonstrate the entire gland. The pancreatic duct is not always seen in normal patients. Usually appearing as a single echogenic line less than 1 mm, it can be located in the pancreatic body in a plane cephalad to the splenic vein.
Developmental and Congenital Anomalies
A very small pancreas (head only) is due to agenesis of the dorsal pancreas during the embryonic stage and is associated with polysplenia. Annular pancreas is the result of a bifid pancreatic head, which encases the duodenum. This anomaly is associated with duodenal atresia or stenosis.
Cystic fibrosis is a recessively inherited disease, with a prevalence of 1 in 3,500 in Caucasians and 1 in 17,000 in African Americans in the United States. Cystic fibrosis affects the exocrine glands in the lungs and GI tract, which produce abnormal highly viscous mucus. Those affected with cystic fibrosis have pancreatic exocrine dysfunction because of obstruction of the small ductules by mucoid secretions. The obstruction of the small ductules leads to subsequent tissue destruction and atrophy and eventual replacement of the pancreatic tissue with fibrosis and fat. Eventually, normal function is compromised, and pancreatic insufficiency results.
In cystic fibrosis patients, the pancreas is quite hyperechoic because of the replacement of normal pancreatic tissue by fibrosis and fatty tissue. Sonographically, apparent cysts may be found. Intraluminal calcifications can be found, but inflammatory changes are not common. Pancreatitis may occur but is not common, and notably, the sonographic appearance is not affected.
Other causes of a hyperechoic pancreas in childhood include steroid therapy, chronic pancreatitis, obstruction of the main pancreatic duct, and Cushing syndrome. Clinical and biochemical tests can distinguish these diseases from cystic fibrosis.
Congenital Cysts48
von Hippel-Lindau disease and autosomal polycystic disease are autosomal dominant disorders that can cause cysts in the pancreas as well as other organs (liver, kidney, adrenal glands, and spleen). Patients with von Hippel-Lindau disease also present with cerebellar, medullary, and spinal hemangioblastomas and pheochromocytomas.
Pancreatic neoplasms are uncommon in childhood, but their clinical appearance is similar to that in adults.
Pancreatic Carcinoma
Pancreatic carcinoma is a nonfunctioning tumor. Early diagnosis is difficult because of the variety and nonspecificity of early signs and symptoms. Because of this, the tumor is often large by the time it is discovered, and metastases to the liver, lymph nodes, and lung have already occurred.
Sonographically, pancreatic carcinoma usually appears as a localized, hypoechoic mass in comparison to the homogeneous texture of the normal pancreas. Focal enlargement is also an important sonographic clue. Lesions less than 2 cm are often difficult to detect, especially if they create only minor acoustic alterations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree