Organ Transplantation
Kevin D. Evans
|
OBJECTIVES
Describe each part of the comprehensive patient history including laboratory values and medications and its importance in evaluating the patient with an organ transplantation.
Describe the clinical presentations, pathologies, and sequelae leading to the need for an organ transplantation.
Differentiate between organ donations from a living donor and one harvested from a cadaver.
Illustrate the most common surgical placements for the renal, pancreas, and liver allografts with a rationale for each location.
Correlate the sonography evaluation of a transplant patient to include the grayscale parenchymal echogenicity, Doppler data, and medical complications associated with pathology or rejection.
Demonstrate completing a diagnostic sonography examination on patients with a renal, pancreas, and/or liver transplant.
Discuss other procedures used to evaluate patients with organ transplants.
KEY TERMS
allograft
Doppler
heterotopically
histocompatibility
perfusion
GLOSSARY
allograft
graft transplanted between genetically nonidentical individuals of the same species
graft transplanted between genetically nonidentical individuals of the same species
histocompatibility
the state of a donor and recipient sharing a sufficient number of histocompatibility antigens so an allograft is accepted and remains functional
the state of a donor and recipient sharing a sufficient number of histocompatibility antigens so an allograft is accepted and remains functional
immunosuppressive medication
pharmaceutical agents prescribed to prevent or decrease the immune response
pharmaceutical agents prescribed to prevent or decrease the immune response
Sonographic evaluation of transplanted organs is a routine exam completed in most general sonography departments. However, a true understanding of the sonographic information and its diagnostic value continues to evolve. As with many sonography examinations, a holistic approach is needed to ensure that the data acquired are placed in context with the many other pieces of diagnostic information obtained on these patients.
COMPREHENSIVE PATIENT HISTORY
Prior to conducting a sonographic examination on a patient with a transplanted organ, it is of utmost importance to obtain a comprehensive patient history. Accomplishing this task will require access to the patient’s electronic medical record so that a thorough search can be conducted to gather information, such as the origin of the native organ disease, site of transplantation, preexisting malignancy or
infections, and any other medical issue that could impact the activity of the organ.1 This information is gathered each time a transplant patient returns to the hospital with a change in their health following a transplant procedure or hospital discharge. Typically, these patients provide an extensive oral history, which needs to be checked against their medical record for accuracy.
infections, and any other medical issue that could impact the activity of the organ.1 This information is gathered each time a transplant patient returns to the hospital with a change in their health following a transplant procedure or hospital discharge. Typically, these patients provide an extensive oral history, which needs to be checked against their medical record for accuracy.
An additional piece needed in making the diagnosis is a review of the patient’s current clinical laboratory values with a focus on the typically most sensitive tests2 (Table 24-1). It is also advised to consult the record for information on the patient’s immune status and any pathology report that might be available. Screening patients for the presence of an infectious disease is also paramount.
As with conducting any sonography examination, it is imperative to spend time evaluating the previous imaging studies in order to form a diagnostic baseline for the current study to be performed. Often, Doppler information and the grayscale dimensions of the transplanted organ can provide important formative information while new information is being gathered. The interpreting physician’s reports, the postoperative notes, and the transplant surgeon’s diagrams cumulatively provide the background material that can prove to be invaluable during the sonographic examination because they will expedite the time spent in examination.
Patients are usually good at self-reporting their medications, and—although valuable to the interpreting physician, this information needs to be verified against their medical record. Most patients will be taking some amount of immunosuppressive medication, with the most common being cyclosporine A (CsA), sirolimus (Rapamycin), or tacrolimus (Prograf), and patients often take some accompanying levels of steroids.2 These drugs must be closely monitored to ensure that they are providing the proper protection because lower levels lead to rejection and higher levels can contribute to toxicity. High-pressure liquid chromatography is considered to be the current gold standard for obtaining quantitative information about drug levels.2 Certainly, these values can be important to the referring physician who has to assemble all the diagnostic information to adjust the patient’s treatment plan.
A comprehensive patient history can assist the sonographer in obtaining and in evaluating the images and Doppler data. Sonography of the transplanted organ is a key part of the diagnostic workup and is a vital part of a holistic plan of action in determining the proper medical course of action.
TABLE 24-1 Sensitive Clinical Laboratory Tests2 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CLINICAL PRESENTATION
A patient diagnosed with chronic renal failure likely undergoes some form of dialysis to reduce nitrogen-containing wastes that have accumulated in the blood stream. A failure to clear nitrogen-containing wastes from the body results in increased blood urea and creatinine. This condition is referred to as uremia. Uremia has a toxic effect on different body systems, such as the gastrointestinal and nervous systems, causes the skin to take on a yellow color, and also causes itching. These physical manifestations are a combination of uremia and developing anemia in the patient. The inability to synthesize erythropoietin, which governs the production of red blood cells, results in the development of anemia.3 Either hemodialysis three times a week or peritoneal dialysis, which is often done at home at night, should assist the patient in reducing uremia and anemia. However, the process is very hard on the cardiovascular system.4
Cirrhosis of a patient, and its poor prognosis, is one condition that leads to liver transplantation.5 A patient is deemed a candidate for liver transplantation when the patient’s underlying disease becomes so threatening that the risk of surgery is less than the continued life expectancy with the native liver. The 1-year survival rate for a liver transplant patient is 87% and the 1-year graft survival rate is 80.3%.1 The causes for liver failure and ultimate transplantation are hepatitis C, alcoholic liver disease, and cryptogenic cirrhosis.1 Those patients with metastatic cancer, active substance abuse, sepsis, and compromised cardiac function are not typical candidates for liver allograft.1 Patients with portal vein thrombosis are considered high risk because it complicates the surgical procedure and results in lower survival rates.
Uncontrolled diabetes manifests in a variety of pathologies, and many type I diabetics are more likely to seek pancreatic allografts. Since diabetics are at risk for chronic renal failure, there is a tendency to advocate for a dual transplant of a kidney and pancreas. A simultaneous pancreas and kidney transplant (SPK) is reportedly 85% successful, whereas a pancreas following a renal transplant (PAK) is only 78% effective and a pancreas transplant alone (PTA) is only 77% effective.2
Successful transplantation of solid organs has dramatically improved in the short term, with first-year rejection rates dropping; however, the long-term survival rates of transplants have remained unchanged—so, more data are needed on long-term survival of transplanted organs. The least amount of data on survival exists for PTA.6
SURGICAL PLACEMENT
The orientation of the transplanted organ anatomy or allograft is very often dictated by the medical condition of the patient at the time of surgical implantation. The term allograft is defined as graft transplanted between genetically nonidentical individuals of the same species.7 The medical condition of the allograft recipient can vary according to the severity of the recipient’s underlying disease.
There are two types of organ donations: donation from a living donor or one harvested from a cadaver. The benefits of matching the donor and recipient are that both the cellular and humoral rejection pathways can be suppressed. The use of immunosuppressive drugs is necessary to avoid these cellular pathways; however, they do not protect the recipient from fungal, viral, and other infections.2 The
postsurgical risks for infection are coupled with the challenge to regulate the immunosuppressive drugs to achieve an optimal balance for the patient.
postsurgical risks for infection are coupled with the challenge to regulate the immunosuppressive drugs to achieve an optimal balance for the patient.
Renal Allograft
Renal allografts are surgically implanted in a superficial placement in either the right or left lower abdomen.4 Although the renal allograft can be placed transperitoneal or intraperitoneal, the surgical preference is an extraperitoneal placement, which is usually in the right iliac fossa.8 Compared with the left iliac fossa, the right iliac fossa is nearer to major vessels and the urinary bladder. If the transplanted kidney is placed heterotopically, the right kidney is transplanted in the left iliac fossa or the left kidney is transplanted in the right iliac fossa.8
En block is a type of harvesting that preserves cadaveric ureters, main renal arteries and veins, segments of the suprarenal and infrarenal arteries and veins, as well as segments of the aorta, and inferior vena cava (IVC).1 In the case of a cadaveric transplant with a donor renal artery and a portion of the aorta, multiple donor arteries are anastomosed end-to-side to the external iliac artery using a Carrel patch.1,8,9 In the case of a living donor transplant harvested with only the main renal artery, the artery is anastomosed either end-to-end to the internal iliac artery or end-to-side to the recipient external iliac artery8 (Fig. 24-1). Hilar fat and adventitia surrounding the ureter are harvested to maximize the blood supply to these areas.1 The ureter is implanted directly into the superolateral wall of the bladder, via ureteroneocystostomy.1 The ureter can also be joined to the native ipsilateral ureter, otherwise known as an ureteroureterostomy.9 Although the health of the recipient is of primary concern, it is also important to be aware of any intrinsic pathology that might be passed on from the donor and donated tissue.
Pancreatic Allograft
The pancreatic allograft is implanted as a whole organ and placed either in the pelvis or the upper abdomen. In the pelvis, the pancreatic allograft is oriented vertically and the arterial anastomosis is made with the iliac artery. The donor’s portal vein is sewn into the external iliac vein and a stump of the donor’s duodenum is inserted so that it empties into the recipient’s urinary bladder.1 In the upper abdomen, the pancreatic allograft is oriented diagonally and this placement attaches the donor’s portal vein to the recipient’s superior mesenteric vein (SMV). The donor’s duodenal stump is sutured into the recipient’s jejunum, which is much like a Roux-en-Y gastric bypass procedure.1
An alternative procedure that is growing in popularity is a pancreatic islet allotransplantation. This alternative procedure is based on the harvesting of pancreatic islets, also called islets of Langerhans. These tiny clusters of cells are scattered throughout the pancreas and the transplantation procedure begins with taking the islets from the pancreas of a deceased organ donor and then purifying, processing, and transferring them into the living donor. The transplantation procedure requires that a physician (typical interventional radiologist) inserts a catheter into the recipient’s portal vein to inject the pancreatic islet cells. The cells are infused, or pushed, slowly into the liver through the catheter. Usually, the patient receives a local anesthetic and a sedative. In some cases, a surgeon performs the transplant using general anesthesia. The purpose is to seed the native pancreas with cells that will spur the organ into proper function and relieve the patient from insulin injections.10
Liver Allograft
The surgery for a liver allograft is quite complex because it requires that four vascular connections as well as a biliary anastomosis be made for proper perfusion and drainage. The hepatic artery is typically anastomosed by either suturing in the donor’s celiac artery to the recipient’s split right and left hepatic or at the branch point of the gastroduodenal and proper hepatic arteries.1 In some cases, an interposition graft must be used to hook the donated celiac axis directly into the recipient’s aorta.
The donated portal vein is end-to-end anastomosed to the recipient’s portal vein. In the situation of a portal vein thrombosis, a jump graft may be needed in order to unite the vessels around the area of thrombosis.
The IVC is transected above and below the donated liver so that these vascular connections can be made with an end-to-end anastomosis with the native IVC. Additional surgical techniques, such as a side-to-side or an end-to-side connection between the donor IVC and the recipient’s IVC, are connections that are likely made between the donor’s IVC and the stump of the recipient’s hepatic veins.
The donor’s bile duct can be united with the recipient’s biliary system by an end-to-end anastomosis after the gallbladder has been removed. A T-tube can be left in place for those patients that have a diseased biliary tree. Those with advanced biliary disease may need a choledochojejunostomy.
This type of allograft is typically provided as a result of a cadaver donation; however, living donations are sometimes made. These partial donations involve a right hepatectomy for segments V, VI, VII, and VIII along with the right hepatic vein.1 But, regardless of the donation source, the anastomotic connections must be carefully interrogated to ensure that the vascular connections are not stenotic and provide adequate perfusion to the allograft.
ALLOGRAFT PHYSIOLOGY
The renal allograft has an average life span of 7 to 10 years; however, this is increased for those who receive a living donor organ to a life span expectancy between 15 and 20 years of function.1,9 Upon transplantation, the renal allograft experiences a margin of hypertrophy of up to 15% in size within the first 2 weeks postsurgically. It is also expected to increase in volume by 40% and maintain its final size and shape about 6 months postoperatively.1
Pancreatic allografts have a reported survival rate of 95% at 1 year and have decreasing rates of acute rejection (AR). As many as 80% of pancreatic transplant recipients are freed from insulin injections after 1 year postoperatively.1 Postoperative monitoring of active rejection is necessary along with increased immunosuppressive therapy to avert ischemia of the allograft.11
Liver allografts should begin functioning immediately and the normal flow anticipated in the hepatic artery is a rapid acceleration of less than 100 ms. The flow should be continuous throughout the diastole with a resistive index (RI) between 0.5 and 0.7.12 The portal vein will have hepatopetal flow into the liver and may appear turbulent. Additionally, the hepatic veins should demonstrate their expected phasic flow that crosses the Doppler baseline owing to changes in the cardiac cycle. Assessing perfusion and determining the patency of the IVC are important factors in predicting the success of the liver transplant procedure.
LABORATORY TEST RESULTS
Renal allografts can be monitored in conjunction with imaging by analyzing biomarkers. The two most frequently monitored during AR are increased levels of blood urea nitrogen (BUN) and creatinine. Alongside these lab tests, the electrolytes for the patient need to be closely monitored for changes. If the rejection episode progresses, the laboratory values for BUN and creatinine will also continue to escalate.13 An estimated glomerular filtration rate (eGFR) can be derived for a kidney transplant, and careful evaluation is needed when the values drop below 10 rnl/rnin.12 A successful renal allograft should have a mean eGFR value of between 50 and 60 mL/hour.14 One of the most popular equations for deriving eGFR in kidney transplant recipients is the Modification of Diet in Renal Disease (MDRD). This formula takes in several factors for calculating the mean GFR (mGFR).15 Research has suggested that a 30% drop in eGFR during the first and third years posttransplantation is associated with a negative outcome for transplant survival.16
The pancreatic allograft provides exocrine secretions of amylase, lipase, and anodal trypsinogen into the bladder, which help to provide biomarkers of acute acinar cellular injury. Elevated levels of amylase and lipase are indicative of inflammation.13 An increase in parenchymal water content is associated with rejection and is thought to be related to a swelling in overall allograft size.17 Blood glucose also provides a measure of endocrine function.
The liver allograft should be monitored for function with biomarkers that are typically used for a native liver. The allograft is expected to function at an optimal level and any decrease in function may be an indicator of tissue ischemia. Sonographic evaluation of the vascular patency of the liver allograft is indicated owing to abnormal liver function biomarkers.
SONOGRAPHIC ANATOMY
Renal Allograft
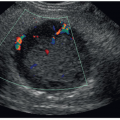
The renal allograft must be evaluated sonographically for its size and overall echogenicity. The grayscale images that are chosen for inclusion in the patient’s record should demonstrate the echogenicity of the cortex as well as the renal sinus. The normal sonographic appearance of the renal cortex is hypoechoic with prominent medullary pyramids that are anechoic. The thickness and the qualitative assessment of the renal allograft cortex will be highly relevant for gauging the potential for rejection and possible ischemia of the tissue. Because the cortex is the primary site for urine production, close attention needs to be paid to this area of the allograft. The renal allograft sinus is normally hyperechoic and contains the hilum for vascular insertion as well as the pelvis for urine excretion. Depending on the stage of transplantation, small amounts of fluid can be visualized within the renal pelvis, especially immediately after the postoperative period. If there is confusion in identifying vessels versus the ureteropelvic junction, power Doppler is a good diagnostic tool to clarify the anatomic difference. Color and power Doppler are the primary diagnostic tools for the evaluation of renal transplants and facilitate a rapid assessment of global renal artery perfusion and venous patency.12 The current technology allows for definitive visualization of the main, anterior, and posterior divisions of the renal artery and vein. Additionally, within the renal sinus and cortex, Doppler allows for the assessment of the segmental, interlobar, and arcuate arteries with the corresponding veins (Fig. 24-2).
The normal renal allograft has a low-resistance vascular bed, which is characterized by streamlined systolic flow and continuous forward flow during diastole. The normal main renal artery has a velocity that ranges between 80 and 118 cm/second.12 As mentioned earlier, the renal allograft will be expected to increase in size during the postsurgical period. Resolution of any fluid collections adjacent to the renal allograft should be carefully documented because these have the potential to compress vital arterial flow to the allograft or venous drainage from the allograft.
Pancreatic Allograft
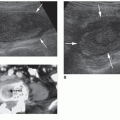
The sonographic appearance of a pancreatic allograft is very similar in echogenicity to that of native pancreas (Fig. 24-3A). The transplant is surgically placed either vertically in the right lower quadrant or diagonally in the upper abdomen for enteric drainage into the portal vein. Regardless of its placement, patency of the vasculature associated with the transplanted pancreas is important to assure proper perfusion of the tissue (Fig. 24-3B). The arterial and venous flow should be carefully documented to determine the potential for resistance. Spectral Doppler should document monophasic venous flow and low-resistant arterial waveforms (Fig. 24-3C, D). The pancreatic duct should also be visualized to ensure that pancreatic digestive enzymes and juices are being generated and are flowing out of the allograft. Extra pancreatic fluid collections should be carefully monitored. Again, the documentation of fluid collections adjacent to the pancreatic allograft has the potential to compress the vascular flow directed into and out of the organ. Bowel gas in the abdomen or pelvis limits the evaluation of the area around the pancreatic allograft.
Liver Allograft
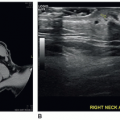
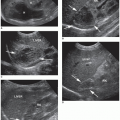
The transplanted liver is in many ways similar in echogenicity to the normal healthy liver (Fig. 24-4A, B). The portal triad of the portal vein, hepatic artery, and bile duct must be
carefully documented to ensure that the surgical anastomosis between the allograft and the native vasculature is patent (Fig. 24-4C-F). Additionally, the hepatic veins must also be evaluated to ensure that they are patent and flowing in the correct direction and draining into the IVC (Fig. 24-4G, H). Doppler waveforms within the hepatic artery, portal vein, and hepatic veins should be recorded to ensure that proper perfusion of the allograft is accomplished. Angle correction of the Doppler cursor to at most 60 degrees is necessary in order to capture accurate and reproducible spectral waveforms of the flow in the segments of vessels interrogated. The sonographic evaluation of the liver allograft is highly dependent on the documentation of flow in the intrahepatic and extrahepatic vessels. Often, if patency and correct direction of flow are not demonstrated, the patient will have more invasive vascular studies and/or will return to the surgical suite. Since narrowing or occlusions within the vasculature are a risk, careful interrogation at points both inside and outside of the liver allograft needs to be made. The portal vein should be sampled in the main, right, and left branches to ensure hepatopetal flow. Owing to the importance of these measurements, careful use of the sonographic equipment is required to provide quick and accurate results.
carefully documented to ensure that the surgical anastomosis between the allograft and the native vasculature is patent (Fig. 24-4C-F). Additionally, the hepatic veins must also be evaluated to ensure that they are patent and flowing in the correct direction and draining into the IVC (Fig. 24-4G, H). Doppler waveforms within the hepatic artery, portal vein, and hepatic veins should be recorded to ensure that proper perfusion of the allograft is accomplished. Angle correction of the Doppler cursor to at most 60 degrees is necessary in order to capture accurate and reproducible spectral waveforms of the flow in the segments of vessels interrogated. The sonographic evaluation of the liver allograft is highly dependent on the documentation of flow in the intrahepatic and extrahepatic vessels. Often, if patency and correct direction of flow are not demonstrated, the patient will have more invasive vascular studies and/or will return to the surgical suite. Since narrowing or occlusions within the vasculature are a risk, careful interrogation at points both inside and outside of the liver allograft needs to be made. The portal vein should be sampled in the main, right, and left branches to ensure hepatopetal flow. Owing to the importance of these measurements, careful use of the sonographic equipment is required to provide quick and accurate results.
 FIGURE 24-4 A, B: Grayscale liver allograft. The echogenicity of the transplanted liver is similar to the native liver. A: The longitudinal image is obtained on a liver allograft transplanted 8 days previously. B: The transverse image of the liver allograft demonstrates normal echogenicity on a patient with an associated right pleural effusion (PE). C-F: Evaluation of portal triad vessels. C: Color Doppler images of turbulent main portal vein of a liver allograft. D: Spectral Doppler tracing of the forward flow into the right branch of the portal vein. E: Spectral Doppler tracing of the main hepatic artery perfusing the liver allograft. F: Spectral Doppler tracing of the left portal vein with forward flow into the left lobe of the allograft. Evaluation of hepatic veins. G: Color Doppler evaluation of the hepatic veins within the liver allograft. H: Spectral tracing of the turbulent flow within the left hepatic vein in the liver allograft.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|