The Prostate Gland
George M. Kennedy-Antillon
|
OBJECTIVES
Discuss embryologic development, differentiation of structures, and hormones influencing maturation of the prostate gland.
Identify surface, relational, and internal prostate anatomy to include differentiating the four prostate zones.
Demonstrate routine scanning procedures to include patient preparation; patient instructions; patient position; transrectal and transabdominal scanning, biopsy techniques; technical considerations; and common scanning pitfalls.
Describe the pathology, etiology, clinical signs, symptoms, and sonographic appearance of cysts in the male pelvis to include Müllerian duct and utricle cysts, seminal vesicle cysts, prostatic cysts (prostatic abscess), and diverticula of the ejaculatory ducts and vas deferens.
Explain the pathology, etiology, clinical signs and symptoms, and sonographic appearance of benign prostatic hyperplasia.
Identify the pathology, etiology, clinical signs and symptoms, and sonographic appearance of prostate calcifications.
Categorize the pathology, etiology, clinical signs and symptoms, and sonographic appearance of prostatitis to include acute bacterial prostatitis, chronic bacterial prostatitis, chronic pelvic pain syndrome, and asymptomatic inflammatory prostatitis.
Recognize sonographic characteristics of benign and malignant conditions of the prostate gland.
Briefly discuss the multiparametric ultrasound (mp-US) approach of combining B-mode and Doppler ultrasound (US) with volume imaging (3D), contrast-enhanced US (CEUS) and shear wave elastography (SWE) to improve the diagnostic performance in detecting prostate cancer (PCa).
Discuss the role of sonography in providing guidance for biopsy procedures.
Discuss the role of sonography in evaluation of suspected male infertility.
KEY TERMS
benign prostatic hyperplasia (BPH)
digital rectal examination (DRE)
infertility
multiparametric ultrasound (mp-US)
neurovascular bundle
prostate biopsy
prostate cancer (PCa)
prostate-specific antigen (PSA)
prostatitis
transrectal ultrasound (TRUS)
transurethral resection of the prostate (TURP)
GLOSSARY
apex
inferior portion of the prostate gland, located superior to the urogenital diaphragm
inferior portion of the prostate gland, located superior to the urogenital diaphragm
base
superior portion of the prostate gland, located below the inferior margin of the urinary bladder
superior portion of the prostate gland, located below the inferior margin of the urinary bladder
corpora amylacea
calcifications commonly seen in the inner gland of the prostate
calcifications commonly seen in the inner gland of the prostate
Eiffel Tower sign
shadowing artifact created in the area of the urethra and verumontanum
shadowing artifact created in the area of the urethra and verumontanum
ejaculatory duct
duct that passes through the central zone and empties into the urethra; originates from the confluence of the vas deferens and the seminal vesicle
duct that passes through the central zone and empties into the urethra; originates from the confluence of the vas deferens and the seminal vesicle
endogenous calculi
calculi formation within the substance of the prostate
calculi formation within the substance of the prostate
exogenous calculi
calculi found in the urethra
calculi found in the urethra
seminal vesicles
paired simple tubular glands that extend from an outpouching of the vas deferens; located superior and posterior to the prostate, between the urinary bladder and rectum
paired simple tubular glands that extend from an outpouching of the vas deferens; located superior and posterior to the prostate, between the urinary bladder and rectum
surgical capsule
a demarcation between the inner gland (central and transitional zones) and outer gland (peripheral zone), which is normally hypoechoic but may be echogenic if corpora amylacea or calcifications are present
a demarcation between the inner gland (central and transitional zones) and outer gland (peripheral zone), which is normally hypoechoic but may be echogenic if corpora amylacea or calcifications are present
vas deferens
reproductive duct that extends from the epididymis to the ejaculatory duct; also known as the ductus deferens
reproductive duct that extends from the epididymis to the ejaculatory duct; also known as the ductus deferens
verumontanum
a longitudinal elevation or ridge of tissue on the posterior prosthetic urethral wall where the orifices of the ejaculatory ducts are located
a longitudinal elevation or ridge of tissue on the posterior prosthetic urethral wall where the orifices of the ejaculatory ducts are located
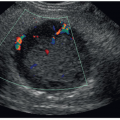
Transrectal ultrasound (TRUS) of the prostate has improved markedly over the past several decades. Equipment technology has progressed from one of the earliest devices, a chair-type apparatus with a mechanical probe mounted in the center,1 to current phased array, three-dimensional (3D) and high-resolution micro-ultrasound transducers (Fig. 14-1). TRUS is utilized to evaluate the prostate in the setting of malignancy, infertility, chronic pelvic pain syndrome (CPPS), and congenital abnormalities. It also plays a significant role in biopsy and treatment guidance.1 The role of contrast agents and elastography in prostate tumor assessment is also being investigated and has shown promising results.1, 2, 3 and 4 Additionally, studies show contrast-enhanced TRUS has a high sensitivity when detecting diffuse prostate cancers that might be missed on a baseline examination. However, current data show contrast-enhanced TRUS alone is not sufficient to confirm or exclude the presence of cancer. Combining the data acquired from individual US modalities into a mp-US approach can improve the diagnostic performance in PCa diagnosis.4,5 Larger trials are necessary to generate more accurate estimates of the sensitivity and specificity of the individual ultrasound modalities.5
A sonography examination of the prostate requires a thorough understanding of the anatomy of the prostate, the sonographic characteristics of disease processes, and the unique image orientation of the transrectal transducer.
ANATOMY AND ORGANOGENESIS
During embryogenesis, all embryos start out with two paired sex ducts: the mesonephric or wolffian ducts and the paramesonephric or müllerian ducts. Each müllerian duct lies lateral to its corresponding wolffian duct.6 This is termed the indifferent stage.6 The mesonephric ducts are responsible for development of the male reproductive system, whereas the paramesonephric ducts will form the female reproductive system. In males, a portion of each mesonephric duct will later develop into an ejaculatory duct, vas deferens, and seminal vesicle. During the 11th gestational week, the prostate begins to form from the urogenital sinus, an endodermal derivative. It begins as multiple solid outgrowths of the prostatic portion of the urethra, which form on the posterior side of the urogenital sinus.6 As development continues, these penetrate the surrounding mesenchyme to form the five prostatic lobes.7 Maturation of the gland continues while testosterone levels are high. Once these levels start to decrease, the gland enters a quiescent state until puberty when testosterone levels rise again.
Gross Anatomy
The adult prostate is a funnel-shaped, exocrine gland surrounded by a fibromuscular capsule. This is not a true capsule, but rather thin, inseparable connective tissue. In the young adult, it weighs approximately 20 ± 6 g and measures on average 4 × 3 × 2 cm. The prostate is composed of glandular and fibromuscular tissue and surrounds the proximal male urethra. The cephalic portion of the gland is referred to as the base and the caudal end the apex. The base is continuous with the bladder neck and the apex is adherent to the urogenital diaphragm (Fig. 14-2A). Three luminal structures traverse the prostate gland: the right and left ejaculatory ducts, and the urethra. The prostatic urethra traverses the central portion of the gland. The duct of the seminal vesicle joins the ampullae of the vas deferens to form the ejaculatory duct at the prostate base. The ejaculatory duct then extends within the central zone
of the gland to the midline where it joins the urethra at the verumontanum, a longitudinal ridge on the posterior wall of the prostatic urethra.1,8 This structure is located at the midpoint of the prostatic urethra near the apex and is laterally flanked by the openings of the ejaculatory ducts (Fig. 14-2B). The utricle, a small epithelium-lined diverticulum at the apex of the verumontanum, is a fetal remnant of the urogenital sinus and is homologous to the female uterus6,8,9 (Fig. 14-2C). Abnormal dilatation, cysts, or calcifications can occur at this level, and they are usually associated with urinary tract symptoms. This structure accounts for the “Eiffel Tower” appearance on transverse images of the prostate gland obtained at this level1,10 (Fig. 14-3).
of the gland to the midline where it joins the urethra at the verumontanum, a longitudinal ridge on the posterior wall of the prostatic urethra.1,8 This structure is located at the midpoint of the prostatic urethra near the apex and is laterally flanked by the openings of the ejaculatory ducts (Fig. 14-2B). The utricle, a small epithelium-lined diverticulum at the apex of the verumontanum, is a fetal remnant of the urogenital sinus and is homologous to the female uterus6,8,9 (Fig. 14-2C). Abnormal dilatation, cysts, or calcifications can occur at this level, and they are usually associated with urinary tract symptoms. This structure accounts for the “Eiffel Tower” appearance on transverse images of the prostate gland obtained at this level1,10 (Fig. 14-3).
Vasculature
The prostate is supplied with blood from the internal iliac arteries via the prostaticovesical arteries.1,11 The iliac arteries course along the medial and inferior surface of the bladder toward the prostate along the neurovascular bundle.12 They bifurcate into the prostatic artery and inferior vesicle artery, which further branch into the urethral and capsular arteries (Fig. 14-4). The capsular vessels are arranged on the surface of the gland in five anterolateral and posterolateral groups. These capsular vessels supply approximately two-thirds of the glandular tissue.11,12 The urethral arteries are directed inward and follow the course of the prostatic urethra, supplying the other one-third of the glandular tissue.11,12
 FIGURE 14-3 Verumontanum. A coronal sonogram of the normal prostate. The “Eiffel Tower” sign (arrow) is seen at the level of the prostatic utricle. |
The venous drainage consists of a prostatic venous plexus, located between the facial layer and capsule (Fig. 14-5). These small veins drain the venous blood from the prostate gland. These vessels join the veins of the bladder and penis to ultimately drain into the internal iliac veins. The internal iliac veins then connect with the vertebral venous plexus, which is thought to be the route of bone metastasis in PCa.
Prostate Zones
The urethra is the primary anatomic reference point within the prostate, dividing the gland into an anterior fibromuscular portion and a posterior glandular portion13 (Fig. 14-6). The glandular tissue accounts for two-thirds of the prostate and contains four zones. Three of the zones—the peripheral zone, the transition zone, and the periurethral zone—have similar embryologic origins from the urogenital sinus, whereas the fourth zone, the central zone, is derived embryologically from the mesonephric ducts.1,7,14 The fibromuscular structures account for one-third of the prostate.
The peripheral zone constitutes about 70% of the glandular tissue of the prostate (Table 14-1). It is located along the posterior, lateral, and apical aspects of the gland, and its ducts drain into the distal segment of the urethra between the verumontanum and the apex. The acini of the peripheral zone are small, round, and simple.14 The peripheral zone is the most common location for carcinomas1,15 and prostatitis to occur, with approximately 70% of prostate cancer (PCa) arising from this area. Sonographically, normal peripheral zone tissue has a homogeneous, isoechoic echotexture. It can be delineated from the central zone by a thin, hyperechoic band separating the two zones. This band, called the surgical capsule, is in the shape of a semicircle and borders the central gland laterally and posteriorly.
The central zone accounts for about 25% of the glandular tissue and is located at the base of the prostate. It is embedded in the funnel-shaped peripheral zone (Fig. 14-7). This zone narrows to an apex at the verumontanum. It surrounds the ejaculatory ducts and is located between the peripheral zone and the transition zone.1,2 Microscopically, the central zone acini contrast strikingly in appearance with those in the peripheral zone. They are large and have irregular contours, with considerable intraluminal folds and ridges.1,14 These morphologic differences are what set the central zone aside from the rest of the glandular tissue16 and what presumably make it immune from most disease.17 The stroma are composed of long, tightly arranged muscle fibers
that sweep around the acini. Only about 5% of prostate carcinomas arise within the central zone. Sonographically, the echogenicity of the central zone is normally brighter than that of the peripheral zone.
that sweep around the acini. Only about 5% of prostate carcinomas arise within the central zone. Sonographically, the echogenicity of the central zone is normally brighter than that of the peripheral zone.
TABLE 14-1 Prostate Zones | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
The transition zone accounts for about 5% of the prostate gland in a young man. It consists of two small lobules found lateral to the proximal urethral segment, and is separated laterally and posteriorly from the outer gland by the surgical capsule (Fig. 14-8). It follows the long axis of the urethra toward the bladder neck.17 The most caudal portion of this zone is at the verumontanum. The acini comprising the transitional zone are similar in appearance to the peripheral zone; however, the stroma is much more compact.1 Being the most common site of involvement by benign prostatic hyperplasia (BPH), the transition zone may account for a much greater percentage of glandular tissue as men age. Also, because the transitional zone is a common location for BPH, sonographically it becomes more heterogeneous and visible. Approximately 20% of carcinomas occur in this area.18
 FIGURE 14-7 A drawing of the funnel-shaped central zone illustrates the ejaculatory ducts’ entrance into the prostate. |
 FIGURE 14-8 This drawing depicts the saddlebag-shaped transitional zone and its relationship to the urethra. |
The periurethral glandular tissue composes less than 1% of all prostatic glandular tissue and is embedded in the smooth muscle wall of the urethra. This region runs along most of the prostatic urethral segment.1 The ducts of this tissue open directly into the urethral lumen. Calculi often occur in this area and are thought to be secondary to reflux of urine into these ducts.
Surrounding Structures
The seminal vesicles are paired saccular structures that lie obliquely and caudally to the prostate. The seminal vesicles
are reservoirs for seminal fluid that fluctuate in size and shape secondary to levels of sexual activity. When void of seminal fluid, they appear as curvilinear, hypoechoic structures that flare out laterally. When these structures fill with seminal fluid, they become large, ovoid-shaped cystic structures. Sonographically, low-level echoes are often appreciated within the anechoic fluid.16,20
are reservoirs for seminal fluid that fluctuate in size and shape secondary to levels of sexual activity. When void of seminal fluid, they appear as curvilinear, hypoechoic structures that flare out laterally. When these structures fill with seminal fluid, they become large, ovoid-shaped cystic structures. Sonographically, low-level echoes are often appreciated within the anechoic fluid.16,20
The ampulla of the vasa deferentia are located adjacent and medially to the seminal vesicles. In cross section, they appear as thick-walled, tubular structures. The ejaculatory ducts are formed at the junction of the ampulla of the vasa deferentia and the seminal vesicles. The prostate’s posterior surface is anterior to the rectum and is separated by connective tissue called the rectovesical septum. The anterior surface is connected on either side to the pubic bone by the puboprostatic ligaments and is separated by a plexus of veins and fat. The anterior portions of the levator ani muscles cover the lateral surfaces of the prostate.
SONOGRAPHIC EXAMINATION
Indications and Contraindications
Indications for performing a sonographic evaluation of the prostate gland are numerous and continually expanding (Table 14-2). TRUS is widely used for anatomic guidance during systematic prostate biopsy. However, sonography alone is not an effective screening tool owing to a reported sensitivity and specificity of between 40% and 59% in detecting the PCa.5,21
TRUS is often utilized in evaluation of patients with an abnormal digital rectal examination (DRE). When a gland feels irregular, sonography is useful in identifying masses, cystic areas, or calcifications. Sonographic characteristics of a disease process may include changes in echogenicity, asymmetry, or distortion of the capsule.22,23 Sonography may also help in identifying nonpalpable lesions. Newer technologies, such as elastography, high-resolution micro-ultrasound and contrast-enhanced ultrasound (CEUS), have been reported to improve the localization and detection of clinically significant PCa.4,5,21,24 These modalities are widely available and desirable owing to a relatively low cost, real-time performance, and lack of ionizing radiation. This concept of mp-US, similar to multiparametric MRI, is gaining interest as a promising approach to PCa imaging.4,5
TABLE 14-2 Clinical Role of Prostatic Evaluation | ||||||
|---|---|---|---|---|---|---|
|
Other important uses of sonography include guidance during biopsy and quantification of prostate size. Ultrasound guidance assists in providing a safe and accurate method of obtaining tissue from a focal area of the gland.25,26 It also allows for accurate biopsy device placement within a specific zone of the prostate.7
A patient presenting with abnormal laboratory values and a negative DRE may also undergo sonographic examination. Prostate-specific antigen (PSA) is a protein specific to the prostatic epithelium.27 The higher the PSA value, the more likely malignancy is present, although it can be elevated in benign processes as well.27,28 PSA is the most common blood test utilized to identify men at increased risk of prostate cancer. However, using PSA alone as a screening is not recommended and remains controversial.28, 29 and 30
Clinical complaints of hematospermia, painful ejaculation, dysuria, and perineal pain may also warrant a prostate sonography examination.
Prostatitis is one of the most common causes of these symptoms. Sonographically, color or power Doppler imaging will show increased vascularity secondary to hyperemia when prostatitis is present. The gland may also appear enlarged and less echogenic than usual. Prostatitis may appear as a focal or diffuse process. Ultrasound is also used to detect BPH, calculi, ejaculatory duct cysts, congenital cysts, or abscesses. Sonographically, BPH will appear as an enlarged nodular inner gland. The echotexture is heterogeneous, and it can contain cystic areas. Calculi may be visualized and appear as echogenic structures that may create a shadow artifact. Cysts and abscess have a spectrum of sonographic appearances ranging from simple and anechoic to more complex collections of fluids and debris.
Infertility is another indication for TRUS. Male infertility may be related to congenital abnormalities such as agenesis or atresia of the seminal vesicles, or ejaculatory duct obstruction secondary to a cyst, mass, or calcification.23
A few contraindications to TRUS do exist. Significant rectal lesions such as fissures, obstructing lesions, thrombosed hemorrhoids, or prostatitis may prevent insertion of the rectal probe owing to patient discomfort.31 Additionally, patients with these symptoms are at increased risk of bleeding or infection.
Scanning Techniques
In the early 1970s, a transabdominal approach to visualize the prostate through a full bladder was utilized (Fig. 14-9). This offered limited information owing to the limited resolution of low-frequency transducers needed to penetrate deep enough to visualize the prostate gland and owing to incomplete visualization of the entire gland. The endorectal approach provides close anatomic proximity to the pelvic organs utilizing a higher-frequency transducer, which results in improved resolution and visualization.
Before beginning a TRUS, pertinent information should be obtained including DRE and PSA results or clinical symptoms associated with infection or BPH.
TRUS should be performed with the patient’s bladder empty to decrease discomfort. The patient is placed in a left lateral decubitus position, with the knees flexed toward the chest similar to the fetal position. The transducer is then properly prepared by placing ultrasound gel within a probe cover, which is then placed over the probe. TRUS probes can be either end-fire or side-fire, and they commonly utilize frequencies of 9 to 12 MHz or higher.21,32 The transducer is inserted within the rectal cavity (Fig. 14-10). When a biopsy is performed, an injection of 2% lidocaine just lateral to the junction of the prostate base and seminal vesicles, at the neurovascular bundle, will significantly reduce pain.
By convention, the orientation of an endorectal scan is slightly different from that of a transabdominal scan and is crucial to understand. The sonography image is inverted so that the near field is at the bottom of the image and the far field is at the top of the image. In the transverse plane, the right lobe of the gland is on the left side of the image and the left lobe of the gland is on the right side of the image. In the sagittal plane, the base of the gland is on the left side of the image and the apex of the gland is on the right side of the image. The rectal wall is in the near field and the urinary bladder is in the far field, with the prostate gland lying between these two structures.
Sonographically, the normal prostate appears as a crescent-shaped structure under the bladder base with an intact capsule. It is symmetrical and surrounds the urethra. Both the shape and echotexture of the prostate should be closely evaluated.
The capsule of the prostate should be smooth and without disruption, and the central gland should be contained within that prostatic capsule. Any disruption of this capsule deserves further exploration for pathology. The internal echo pattern is evaluated by slowly scanning through the gland beginning in a transverse plane at the base of the prostate and moving inferiorly toward the apex. If a lesion is seen within the gland, it is important to discern where the lesion is located.
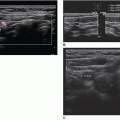
Images are acquired in a transverse plane of the base, mid, and apical regions of the prostate. The base of the prostate is located superior to the verumontanum and has a half-moon shape (Fig. 14-11A). It has a homogeneous echotexture and should be isoechoic to surrounding tissue. The base of the prostate is predominately made up of the central zone. A hypoechoic arcuate line may be seen within the central portion of the base, which represents the surgical capsule. This demarcates the central gland from the peripheral gland.
The transducer is then moved slightly inferior to the level of the verumontanum, which is seen sonographically as a centrally located shadow (Fig. 14-11B). At this level, the urethra passes through the prostate and more of the central gland can be seen. The central gland appears slightly more hyperechoic than the surrounding peripheral gland. This is the level at which the prostate should be measured in both transverse and anteroposterior dimensions. In patients over age 40 years, the central gland may bulge anteriorly owing to BPH, which may change the prostate shape to an ovoid structure.
Located inferior to the verumontanum is the apex of the prostate (Fig. 14-11C). This section is typically more circular and is predominately made up of the peripheral zone. It appears slightly more heterogeneous than the homogeneous base. Because 70% of all prostate cancers occur in the peripheral zone, this is a common location for malignant lesions.
The transducer is then rotated 90 degrees to a sagittal plane. On a midline sagittal image, the urethra can be followed coursing from the bladder neck, through the prostate gland, and toward the apex (Fig. 14-12A). As the transducer is moved to the left or right, more of the central gland comes into view. At this level, the ejaculatory ducts may be visualized. As the transducer is obliqued further, the peripheral zone comes into view (Fig. 14-12B).
 FIGURE 14-12 Sagittal sonogram of the normal prostate. A: The midline section shows the urethra (arrow) coursing through the gland. B: The right lobe of the prostate can be identified. |
The seminal vesicles and vasa deferentia should be imaged in any complete prostate evaluation. These structures are evaluated in both transverse and sagittal planes. The seminal vesicles lay laterally and superior to the base of the prostate. They should appear hypoechoic (Fig. 14-13). If the seminal vesicles appear enlarged, further investigation is required to rule out an obstructive process (Fig. 14-14A, B). The vasa deferentia are visualized in between the seminal vesicles. They are thick-walled and enter the base of the prostate (Fig. 14-15A, B). In a transverse plane, they appear as two donut-shaped structures, and in a sagittal plane, they appear as long tubular structures. The vas deferentia should be free of fluid in the normal setting.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree