The Budd-Chiari Syndrome
The Budd-Chiari syndrome is a relatively uncommon illness that presents with clinical findings of portal hypertension, inferior vena cava (IVC) hypertension, or both as a result of hepatic venous or IVC outflow obstruction. The obstruction may be due to membranous web(s) of the hepatic vein(s) (Fig. 7–1) or suprahepatic IVC, thrombosis of the hepatic vein(s) or suprahepatic IVC, or venoocclusive disease of the liver.1 The most common etiology worldwide for the Budd-Chiari syndrome is membranous obstruction of the hepatic veins or suprahepatic IVC. This condition is seen primarily in persons of Asiatic descent. It is unclear as to whether these webs are congenital in origin or an acquired phenomenon.2 Without aggressive medical, interventional, and surgical care, hepatic vein obstruction can lead to progressive liver dysfunction and death.
In the United States, hepatic vein or suprahepatic IVC thrombosis is the most common cause of the Budd-Chiari syndrome. A large variety of predisposing factors and diseases is associated with the Budd-Chiari syndrome, including: polycythemia rubra vera; paroxysmal nocturnal hemoglobinuria; systemic lupus erythematosus; idiopathic thrombocytopenia; oral contraceptive and estrogen use; pregnancy and the postpartum state; erythema nodosum; juvenile rheumatoid arthritis; Behcet’s disease; primary proliferative polycythemia, myelofibrosisl; primary erythrocytosis; essential thrombocytosis; protein C and S deficiency; antithrombin III deficiency; circulating lupus anticoagulant; malignant tumors of the liver, adrenals, kidneys, and IVC; trauma; and parasitic infection, for example, schistosomiasis.1,3–7 An unusually high incidence of positive hepatitis B surface antigen has been documented in patients who develop hepatic vein or retrohepatic IVC obstruction, suggesting that both hepatitis and preexisting cirrhosis may be risk factors.1,8–10
Venoocclusive disease (VOD) of the liver, the most infrequently reported etiology of the Budd-Chiari syndrome, may result following consumption of herbal teas containing pyrrolizidine alkaloids found within the Heliotropium, Senecio, and Crotalaira species, and comfrey leaves and roots.11 VOD of the liver may occur in 20 to 50% of patients receiving both chemotherapy (i.e., cyclophosphamide, busulfan, carboplatin, methotrexate) and radiation therapy (1200–1440 cGy) before undergoing allogenic bone marrow transplantation.12–15 The mortality rate in this group can be as high as 53%.16 In addition, both a large- and small-vessel thrombosis and VOD of the liver may be seen in 3% of the patients receiving the chemotherapeutic agent dacarbazine.17 Previously, most patients who developed the Budd-Chiari syndrome secondary to venous thrombosis were thought to have no predisposing illness; however, more recent studies have shown a relatively low incidence of idiopathic Budd-Chiari syndrome ranging from 0 to 30%.1,4,18
 Clinical Presentation
Clinical Presentation
The clinical presentation of patients with the Budd-Chiari syndrome is variable. Most patients present with rapid onset of ascites, increasing abdominal girth, and tender liver enlargement; however, some patients may experience a sudden and insidious onset of symptoms, which gradually increase in severity over a period of months to years.19,20 The incidence of ascites, which is often massive, ranges from 83 to 92%.1,3,5,20 Hepatomegaly and splenomegaly secondary to marked congestion are seen in 60 to 100% and 27% of patients, respectively.4,6,21 Generalized abdominal pain (42–100%), abdominal swelling (50–9-3%), splenomegaly (50–99%), jaundice (17–27%), vomiting with or without hematemesis (27–50%), and edema (8–50%) also occur with significant frequency.1,4,5,22 Bleeding varices (9%), edematous lower extremities (4%), and prominent abdominal wall veins (4%) also are reported.21 In one study, the average interval between the onset of symptoms and treatment was 7.7 ± 6 months (range, 1–24 months).21
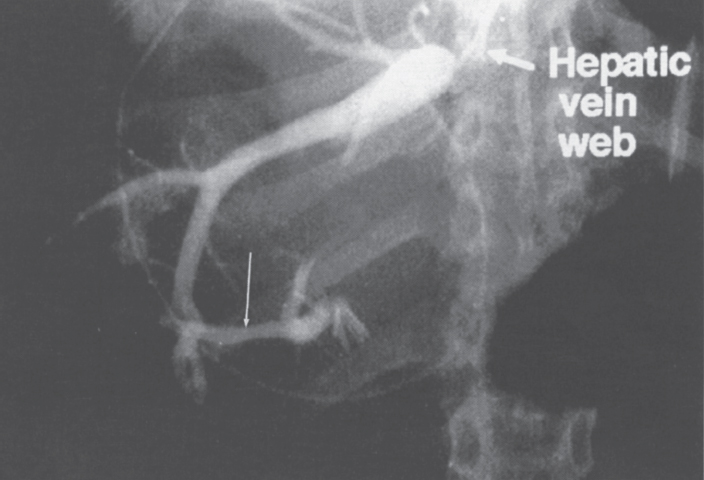
FIGURE 7–1. Selective hepatic venogram shows opacification of the hepatic vein without drainage into the inferior vena cava secondary to the presence of a web. Note the intrahepatic vein to intrahepatic vein communication (long arrow). (Reproduced with permission from Savader SJ. Budd-Chiari syndrome. SCVIR Videodisc II—Portal Hypertension. Options for Diagnosis and Treatment, 1993.)
If the Budd-Chiari syndrome remains untreated or goes unrecognized, progressive portal hypertension will result in esophageal variceal hemorrhage (19–53%), increasing liver dysfunction and coagulopathy, with eventual end-stage hepatic failure, encephalopathy, and death.1,3,5 A few patients may present initially with fulminate hepatic failure and encephalopathy, reflecting overwhelming liver necrosis and gross organ dysfunction.23 Although at least one report has appeared of spontaneous resolution of the Budd-Chiari syndrome in a patient with hepatic vein thrombosis who received conservative medical management, this phenomenon is an exceedingly rare.24
At initial presentation, most patients with the Budd-Chiari syndrome demonstrate little hepatic dysfunction. Laboratory evaluation of serum transaminases, alkaline phosphatase, bilirubin, and prothrombin time in most patients will demonstrate normal to only mildly elevated values. Serum albumin may be decreased, reflecting both impaired hepatic synthesis and loss into the ascites.1,3,25 Quantitative hepatocyte function as measured by galactose elimination capacity and liver blood flow, also tends to remain in the normal range.25 The nonspecificity of the laboratory findings is such that they are not truly helpful in arriving at the diagnosis or predicting the clinical outcome.
 Radiographic Evaluation and Diagnosis
Radiographic Evaluation and Diagnosis
Initial evaluation of patients with suspected Budd-Chiari syndrome is usually noninvasive in nature. An algorithm for the workup and diagnostic evaluation of the Budd-Chiari syndrome is shown in Figure 7–2. Magnetic resonance imaging (MRI) has proven a valuable imaging modality in the evaluation of patients with suspected Budd-Chiari syndrome because of its noninvasive nature, its ability to detect blood flow, and its ability to provide multiplanar imaging of the abdomen and liver. MRI can depict the hepatomegaly, inhomogeneous liver enhancement (particularly on T2-weighted images), varices, and ascites typically seen in this group of patients. Stark et al26 also described multiple-specific MRI abnormalities, which included a marked reduction in caliber or complete absence of the hepatic veins (Fig. 7–3), “comma-shaped” intrahepatic collateral vessels (Fig. 7–4), or significant constriction of the intrahepatic IVC (Fig. 7–5). Stark et al further reported that high-quality imaging could be produced with a T1-weighted spin-echo pulse sequence (time to “relaxation”/time to “echo” 260/18), resulting in a reduction of respiratory, cardiac, and peristaltic motion artifacts.
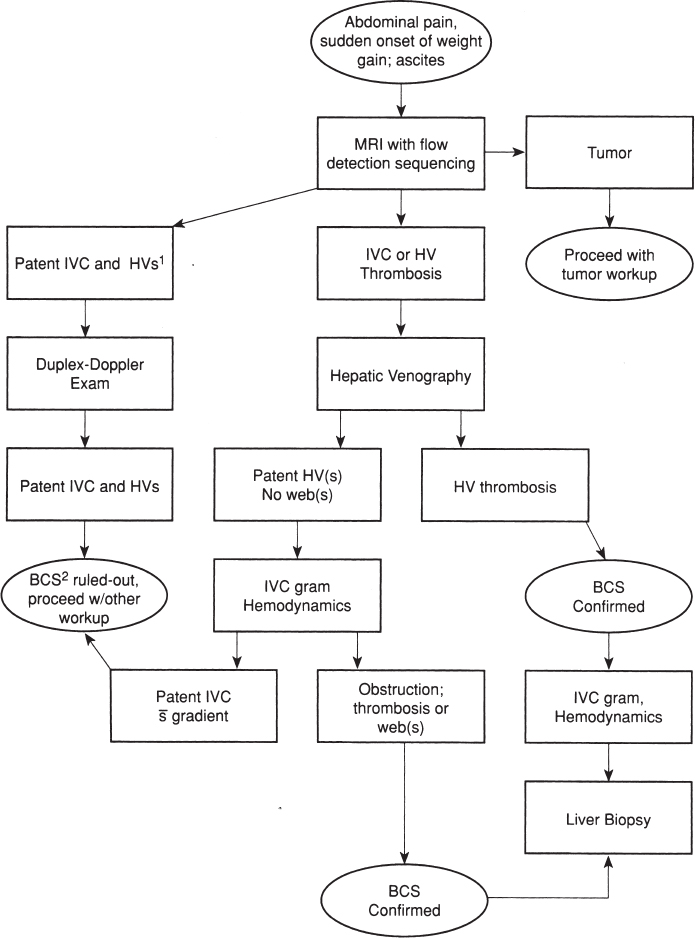
FIGURE 7–2. Algorithm for the work-up and evaluation of the Budd-Chiari syndrome. Note:1HV(s), hepatic vein(s);2BCS, Budd-Chiari syndrome.
If the Budd-Chiari syndrome is secondary to a membranous web, a small, thin, curvlinear soft-tissue membrane or an obliterated segment of IVC or hepatic vein can visualized effectively by T2-weighted spin-echo imaging (TR/TE 2000 msec/60–150 msec).27 Stenoses within the hepatic veins or at their communication with the IVC also can be delineated (Fig. 7–6). Slow flow or thrombus may be visualized within the hepatic veins and IVC, the hepatic veins may appear disconnected from the IVC, or the hepatic veins simply may not be visualized, indicating thrombosis.27–29 Collateral vessels, including the azygous, hemizygous, and superficial abdominal wall veins, are commonly visualized.27 On T2-weighted images or even-echo rephasing, the thrombus typically stands out as a region of high signal intensity against the low-signal intensity background of flowing blood. In an Italian study, MRI was used to evaluate 14 cases of suspected Budd-Chiari syndrome after failure of Doppler ultrasound to yield a diagnosis a definitive diagnosis. MRI demonstrated 14 (74%) cases of major hepatic vein thrombosis, three (16%) cases of membranous hepatic vein obstruction, and one (5%) case of hepatic vein thrombosis plus IVC compression and membranous obstruction of the IVC.28 Compression of the FVC also is clearly visualized. The portal vein may appear compressed, and peripheral branches may not be visualized. In addition, collateral intrahepatic venous circulation and parenchymal inhomogeneity, reflecting congestion, portal blood flow inversion, ischemia, infarction, necrosis, or fibrosis may be demonstrated.27
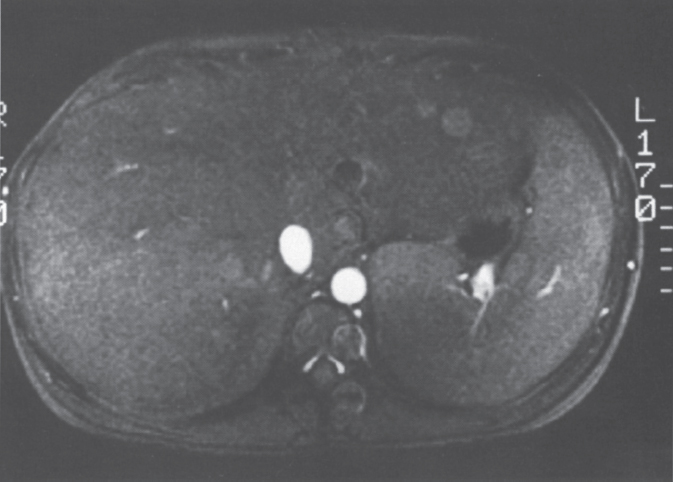
FIGURE 7–3. Axial gradient echo (TR-22, TE–13) image through the liver demonstrates an absence of the hepatic veins.
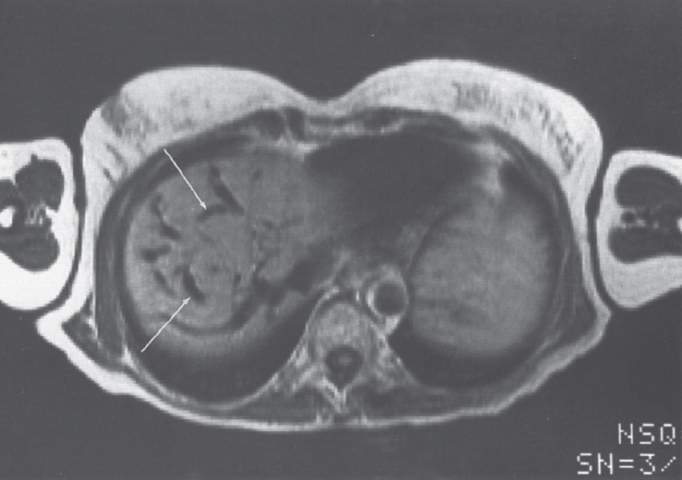
FIGURE 7–4. Axial T2 proton-density (TR-2090, TE-45) image through the liver demonstrates multiple dilated “comma-shaped” structures (arrows) consistent with hepatic vein-to-hepatic vein collaterals.
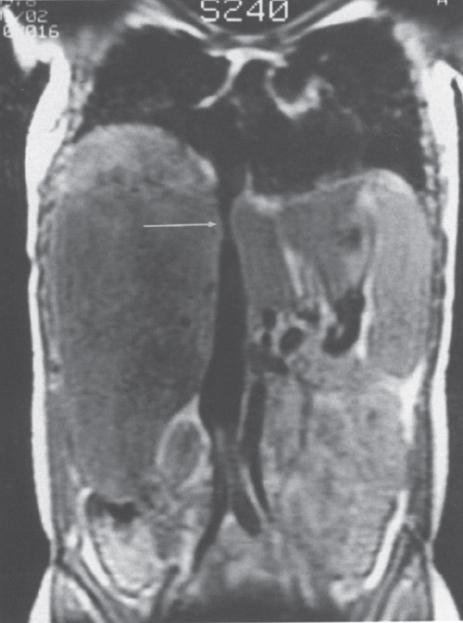
FIGURE 7–5. Spin-echo T1-weighted (TR-800, TE-20) image demonstrates a constriction of the intrahepatic portion of the inferior vena cava (arrow). (Reproduced with permission from Savader SJ. Budd-Chiari syndrome. SCVIR Videodisc II—Portal Hypertension. Options for Diagnosis and Treatment, 1993.)
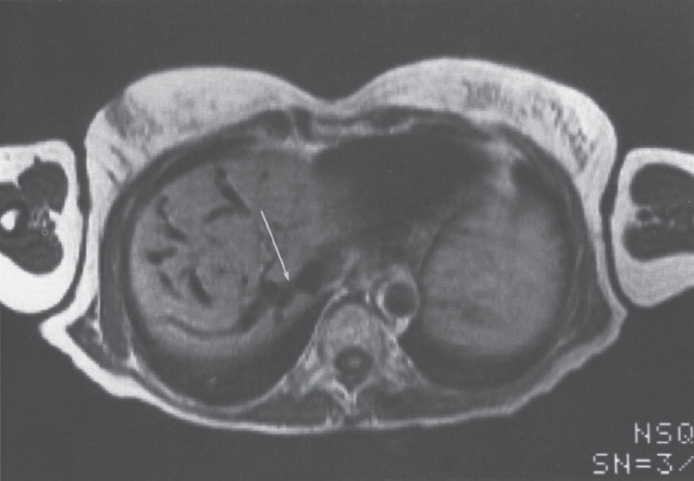
FIGURE 7–6. Axial spin-echo T1 weighted (TR-638, TE-38) image demonstrates a high-grade stenosis (arrow) at the confluence of the right hepatic vein and inferior vena cava.
Many authors have reported that color-flow and duplex-Doppler ultrasound allow for an accurate evaluation in the diagnosis of the Budd-Chiari syndrome. Sonographically, the three major hepatic veins are identified easily in normal patients. In the acute phase of the disease, at least one of the major veins can be identified and typically will demonstrate wall thickening, stenosis irregularity, obstruction, intrahepatic collateralization, and extrahepatic anastomoses (Fig. 7–7).30,31 In the chronic state of Budd-Chiari syndrome, it may be impossible to identify any of the main hepatic veins (Fig. 7–8). The caudate lobe veins may be dilated.30 The IVC may be narrowed, may be shifted to the right secondary to caudate lobe hypertrophy, or may demonstrate intraluminal thrombus. Color-flow duplex ultrasound may be used to evaluate flow characteristics and direction. The slow triphasic signal obtained in the normal hepatic vein and IVC responds to both the cardiac and respiratory cycles. In the Budd-Chiari patient, blood flow signals may be absent, reversed, turbulent, or contiguous.32 Dilatation of a hepatic vein or the IVC without any changes in caliber during dynamic respiratory activity (Valsalva maneuver) is an indirect sign of an obstruction such as a web.30,31 Portal vein flow may be slowed, reversed, or even increased secondary to portal vein to hepatic vein shunting (Fig. 7–9).32
The liver congestion and hepatomegaly characteristics of these patients may result in hepatic vein compression without loss of patency, rendering hepatic veins nonvisible to real-time ultrasound. Color-flow Doppler ultrasound, however, affords an overview of all the real- time events within the scanning sector with the addition of an auditory Doppler signal that can be used to search for a patent hepatic vein. Thus, some invesetigators advocate the use of color-flow Doppler ultrasound over real-time ultrasound alone in the belief that this method of evaluation provides considerably more diagnostic information. Using color-flow Doppler ultrasound, direct hepatic vein visualization is not absolutely necessary because hepatic vein flow can be based on the characteristic auditory signal.33
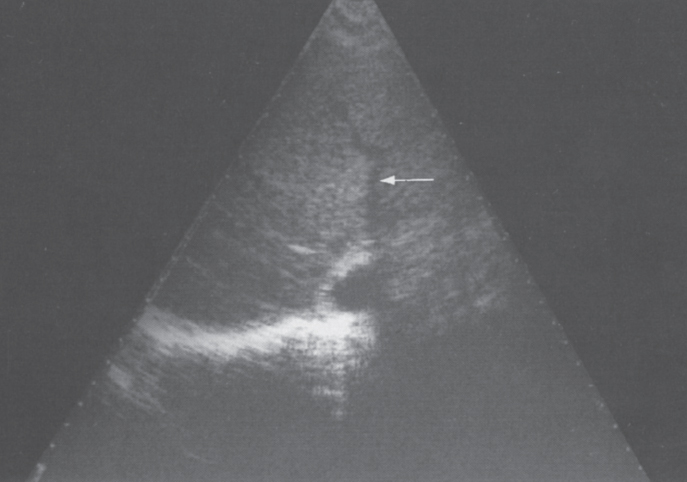
FIGURE 7–7. Ultrasound demonstration of a patent right hepatic vein (arrow) with narrowing and irregularity in patient with acute Budd-Chiari syndrome.
Some of the limitations of sonography include the inability to image webs consistently, underestimation of thrombus or tumor extent, overlying bowel gas artifacts, and operator dependency in determination of Doppler blood flow analysis. Advantages include its noninvasive nature, ability to demonstrate the total length of an obstruction in one image, multiplanar imaging capabilities, availability, and relatively low cost.
Radiocolloid liver scans may be performed with99m-technetium sulphur colloid or 99m-technetium phytate.34 Early in the disease, the study may be normal or may demonstrate only minimal hepatocyte dysfunction. An abnormal scan will show an enlarged liver with inhomogeneous uptake (Fig. 7–10). As the disease progresses, right and left lobe congestion increases and function decreases, with a subsequent increase in the size and activity of the caudate lobe. A caudate lobe “hot spot” is explained by the independent venous drainage into the IVC, which is typically spared from thrombotic occlusion, even when the three main hepatic veins are involved. Unfortunately, this classic appearance may be seen in only 17 to 63% of patients.34,35
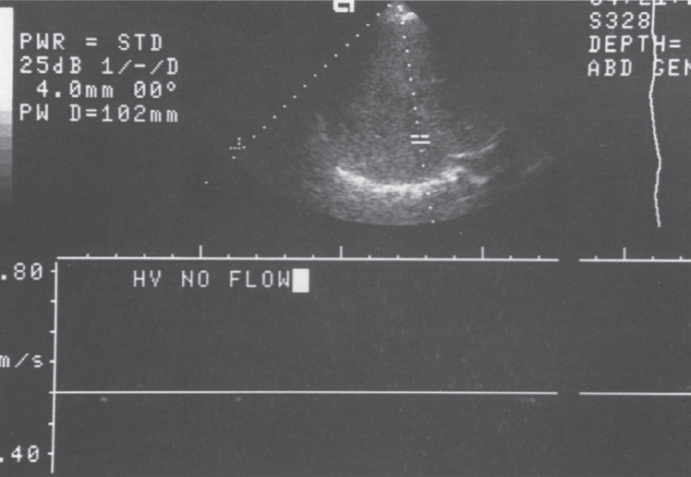
FIGURE 7–8. Duplex Doppler image of the liver demonstrates no flow during sampling of the hepatic vein. (Reproduced with permission from Savader SJ. Budd-Chiari syndrome. SCVIR Videodisc II—Portal Hypertension. Options for Diagnosis and Treatment, 1993.)
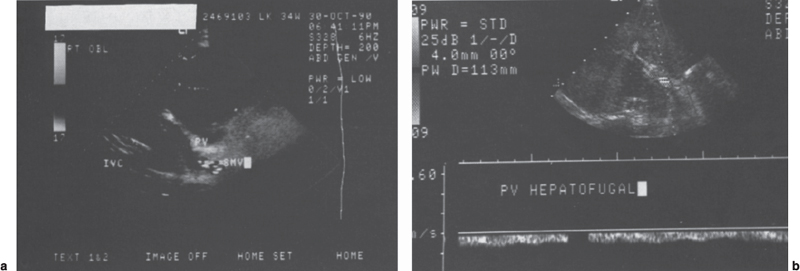
FIGURE 7–9. (a) Color-flow Doppler image of the liver demonstrates a patent portal vein (PV) and superior mesenteric vein (SMV); however, the color coding is consistent with hepatofugal flow. (b) Duplex Doppler image with sampling of the portal vein confirms the presence of hepatofugal flow.
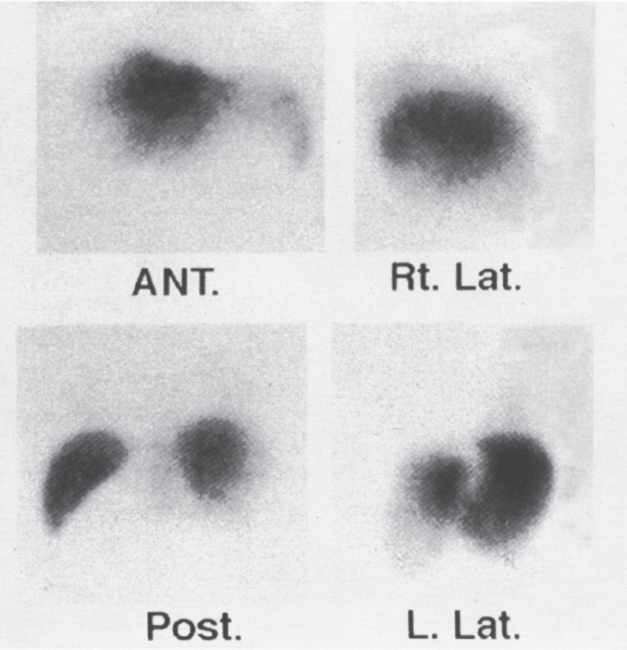
FIGURE 7–10. 99m-Technetium sulphur-colloid liver spleen scan in patient with Budd-Chiari syndrome demonstrates hepatomegaly with inhomogeneous uptake and tracer distribution. (Reproduced with permission from Savader SJ. Budd-Chiari syndrome. SCVIR Videodisc II—Portal Hypertension. Options for Diagnosis and Treatment, 1993.)
If one or two hepatic veins remain patent, these areas of the liver can demonstrate normal hepatic uptake of the tracer. If the IVC is occluded, a pattern of diffuse and symmetric decreased uptake with increased splenic and medullary uptake can present a pattern indistinguishable from hepatic cirrhosis. If multiple sets of accessory hepatic veins are present, hepatic venous outflow can be relatively normal despite occlusion of all three main veins. The scan can therefore appear relatively normal.34
Single-photon emission computed tomography (SPECT) is a process in which axial, sagittal, or coronal images are obtained during a liver-spleen scan using computer reconstruction. Using SPECT techniques, the contribution of counts from the hepatic parenchyma surrounding the caudate lobe can be eliminated, which affords improved visualization of the caudate lobe compared with routine planar imaging. Thus, this technique offers an enhanced degree of sensitivity versus standard nuclear medicine imaging techniques.36 With newer noninvasive, flow-sensitive imaging modalities, such as MRI and duplex—Doppler ultrasound, nuclear medicine techniques, including SPECT, are rarely required for a diagnosis.
The classic computed tomographic (CT) findings of the Budd-Chiari syndrome include hepatomegaly and ascites. On a contrast-enhanced image, two important additional findings are noted: (1) a patchy, inhomogeneous contrast enhancement pattern of the liver with a fan-shaped area of greatest enhancement decreasing towards the periphery (Fig. 7–11) and (2) nonvisualization of the hepatic veins.37–39 The inhomogeneous enhancement is thought to be due to slow flow, venous outflow obstruction, and collateral circulation within the liver. If the enhancement of the liver is plotted over time during a dynamic scan, the pattern is typical of a cavernous hemangioma and markedly different from that seen within the normal liver.38 In addition, the intrahepatic IVC may be compressed (see Fig. 7–11) by an enlarged caudate lobe, and gastroesophageal and splenic hilar varices may be visualized. Right hepatic lobe atrophy may be present later in the disease. Arora et al40 described an unusual CT appearance for the Budd-Chiari syndrome in which multiple low-density inhomogeneous space occupying lesions were present within the liver. These proved to be areas of hemorrhagic necrosis without evidence of malignancy. Noninvasive studies are most helpful in confirming the diagnosis of the Budd-Chiari syndrome when the findings from multiple noninvasive modalities are combined.
Celiac angiography is generally nonspecific. The hepatic arteries may have a stretched and splayed appearance as a result of congestive hepatomegaly (Fig. 7–12A). The parenchymal phase is inhomogeneous with areas of irregular contrast density similar to that appreciated on the contrast-enhanced CT scan. Splenomegaly also may be present, and the splenic artery may be enlarged and unusually tortuous secondary to the increased blood flow. As the chronicity of the disease increases, the angiographic picture becomes similar to that seen in patients with cirrhosis and portal hypertension. Varices are commonly visualized, and portal vein blood flow can become slowed, stagnant, or reversed.39 Portal venous blood may be shunted to the caudate lobe as a result of its autonomous venous drainage, which is usually spared (Fig. 7–12B). Hepatic artery to portal vein shunting41 and intrahepatic portosystemic collaterals also may be present (Fig. 7–13). Mahmoud et al7 also reported the relatively common occurrence of concomitant thrombosis of major branches of the portal venous system, including the portal vein, superior mesenteric vein, and splenic vein, in addition to IVC thrombosis.
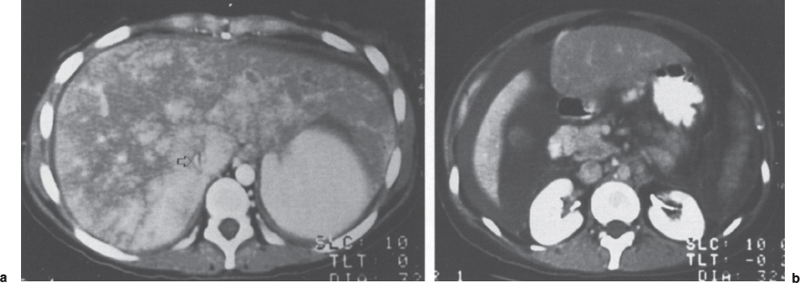
FIGURE 7–11. (a) Axial contrast-enhanced CT image obtained through the liver in this Budd-Chiari syndrome patient demonstrates the classic central fan-shape area of enhancement which decreases toward the periphery. Note the compressed slitlike inferior vena cava (arrow). (b) Note the extensive ascites surrounding the inferior portion of the liver. (Reproduced with permission from Savader SJ. Budd-Chiari syndrome. SCVIR Videodisc II—Portal Hypertension. Options for Diagnosis and Treatment, 1993.)
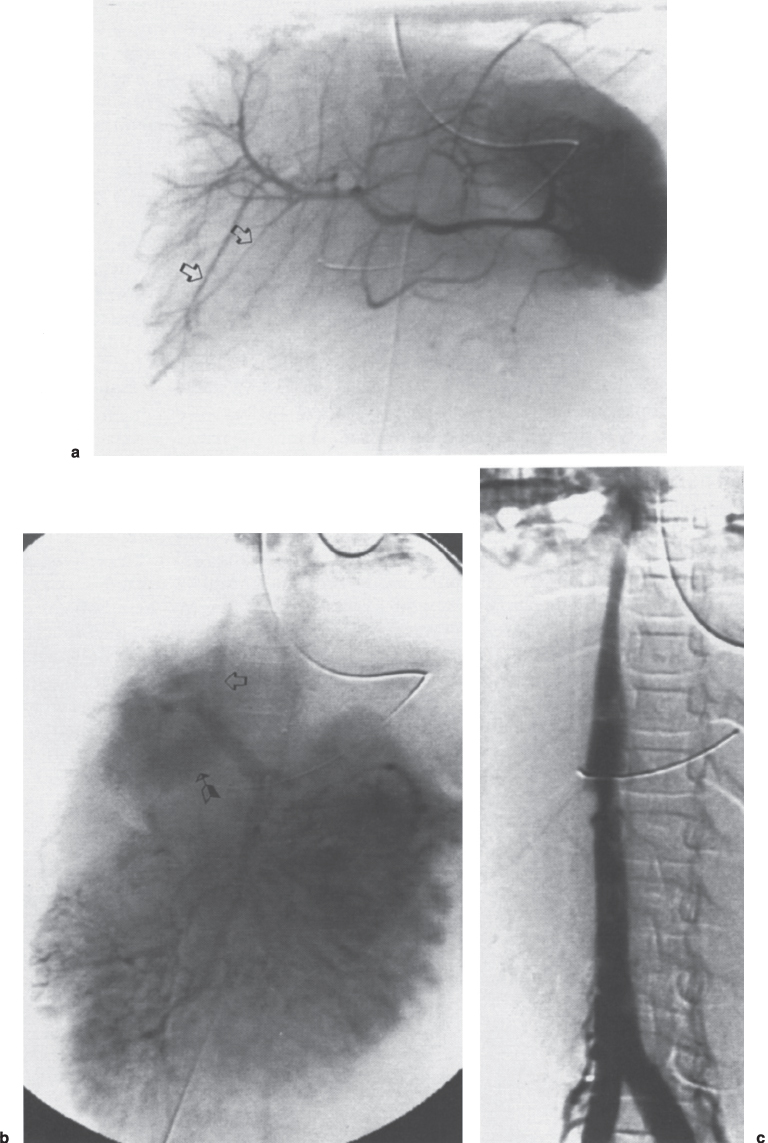
FIGURE 7–12. Budd-Chiari syndrome secondary to blunt abdominal trauma in a 6-year-old child. (a) Celiac axis arteriogram. Note the stretched appearance of the hepatic artery branches (arrows) caused by the congestive hepatomegaly. (b) The portal venous phase of the superior mesenteric artery arteriogram demonstrates shunting of the blood to the caudate lobe (arrow) with drainage directly into the inferior vena cava (open arrow). (c) The inferior venacavogram demonstrates the classic “steeple-shaped” narrowing of the intrahepatic segment of the inferior vena cava.
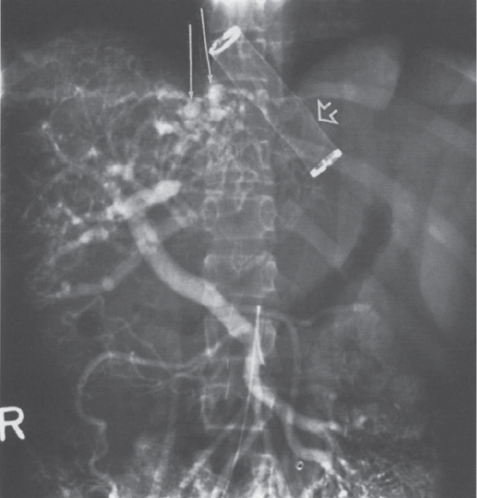
FIGURE 7–13. Multiple intrahepatic portosystemic collaterals (arrows) are appreciated during the portal venous phase of the superior mesenteric artery arteriogram. Note the support tube (open arrow) used to prevent collapse of the mesoatrial shunt as it passes through the diaphragm. (Reproduced with permission from Savader SJ. Budd-Chiari syndrome. SCVIR Videodisc II—Portal Hypertension. Options for Diagnosis and Treatment, 1993.)
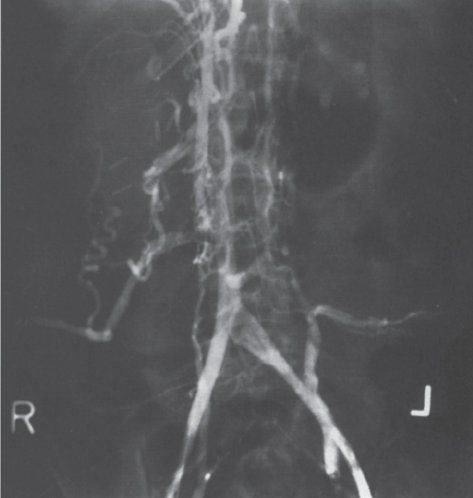
FIGURE 7–14. Thrombosis of the inferior vena cava secondary to caudate lobe compression in Budd-Chiari patient. Note the extensive collateral blood flow through the azygos vein, paravertebral venous plexus, and retroperitoneal collaterals. (Reproduced with permission from Savader SJ. Budd-Chiari syndrome. SCVIR Videodisc II—Portal Hypertension. Options for Diagnosis and Treatment, 1993.)
Hepatic venography remains the gold standard for the diagnosis of the Budd-Chiari syndrome. During venographic evaluation, inferior vena cavography should initially be performed. The inferior vena cavogram classically shows the intrahepatic IVC to be “pointed” or to have a “steeple-shaped” configuration (see Fig. 7–12B). If the IVC is occluded completely, retroperitoneal collaterals, including the hemiazygous, azygous, and paravertebral venous plexus, may be visualized (Fig. 7–14). The subhepatic IVC may be normal in caliber or dilated. In addition, stenoses, membranous obstruction, or a thrombus involving the retrohepatic and suprahepatic segments of the IVC can be demonstrated. In patients with an IVC obstruction as the cause of their syndrome, simultaneous suprahepatic and infrahepatic contrast injections should be used along with pressure measurements to demonstrate the length and hemodynamic significance, respectively, of the occluded segment.42 (Ultrasound and MRI usually can evaluate the length of the obstruction quite accurately.) Tumors originating from the IVC or tumor extension from the kidneys, adrenals, or liver can be identified as an irregular filling defect within the IVC. The filling defect can extend as far superiorly as the right atrium. The right atrial and IVC pressures (suprahepatic, intrahepatic, and infrahepatic levels) should be obtained to evaluate more fully and to assess objectively the degree of caval compression. This information is of the utmost importance in determining which decompressive shunting procedure will best serve the patient’s needs.
Following vena cavography, hepatic venography is performed from the common femoral vein approach using a Cobra or sidewinder-type catheter. A transjugular or basilic vein approach utilizing a hockey stick or multipurpose catheter may be used in cases of confirmed IVC obstruction, high-grade stenosis, or to simplify the procedure in general. Direct angiographic evaluation may demonstrate thrombus in the hepatic vein(s) with occlusion in the most acute phase of the disease (Fig. 7–15). As the Budd-Chiari syndrome progresses, the more typical spiderweb appearance of partially recanalized and collateralized vessels is visualized (Fig. 7–16).
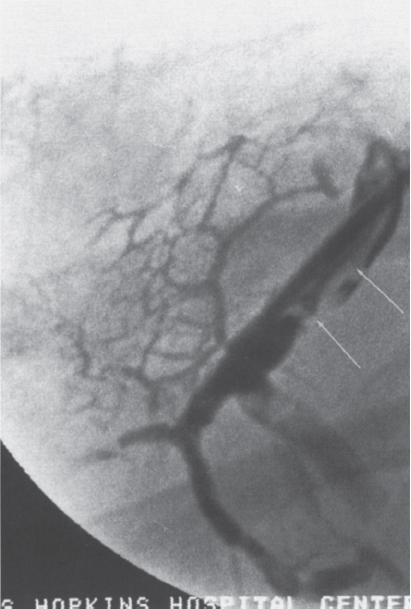
FIGURE 7–15. The hepatic venogram shows a large filling defect (arrows) within the hepatic vein consistent with thrombus. Note the multiple adjacent collateral vessels.
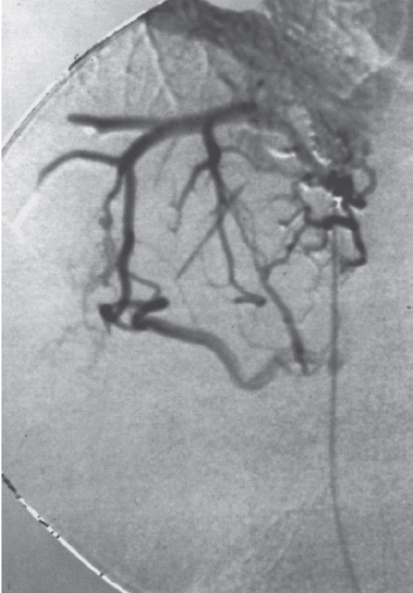
FIGURE 7–16. Classic “spiderweb” appearance of recanalized hepatic veins in Budd-Chiari patient.
If the hepatic veins cannot be selectively catheterized, two options remain. First, a percutaneous transhepatic contrast injection into the liver with a 22-gauge Chiba needle can demonstrate the spiderweb collateral venous pattern. As a second option, color-flow Doppler ultrasound can be used to search for a patent hepatic vein. Percutaneous access can then be obtained under direct visualization. In patients in which a hepatic vein web is present, the hepatic vein typically remains patent. Percutaneous transhepatic venography (with or without ultrasound guidance) using a 22- or 20-gauge needle can be used to complete the diagnostic evaluation. In these cases, pressure measurements obtained on both sides of the web should be used to guide treatment and evaluate the results.
Following radiologic evaluation and confirmation of the Budd-Chiari syndrome, a liver biopsy is required for planning the appropriate treatment and can be performed in patients who have portal hypertension and ascites; the complication rate of liver biopsy is acceptable.1 Patients with a coagulopathy should have this corrected before biopsy is taken. If this is not possible, a percutaneous transjugular liver biopsy or percutaneous transhepatic biopsy followed by Gelfoam embolization of the tract should be performed. Early histologic findings include sinusoidal dilatation with centrolobular congestion. Later, hepatocyte atrophy and necrosis may be present. Depending on the degree of cirrhosis present, variable stages of fibrosis also may be seen as a feature of repair in the injured liver.3,25 In a study of 44 patients with Budd-Chiari syndrome, histologic analysis was available in 31 (70%). The following histologic patterns were noted: (1) sinusoidal dilitation with perivenula hemorrhage (n = 18, 58%); (2) findings in (1) plus variable grades of fibrosis (n = 9, 29%); and (3) cirrhosis (n=4,13%).7
 Interventional Radiology Management Options and Results
Interventional Radiology Management Options and Results
Percutaneous Transluminal Angioplasty
An algorithm for the primary treatment of the Budd-Chiari syndrome including interventional options is shown in Figure 7–17. Percutaneous transluminal angioplasty (PTA) can be used as the primary treatment of Budd-Chiari syndrome for patients with (1) focal and nonfocal stenosis of the hepatic vein(s) or IVC resulting from either congenital or postinflammatory web(s); and (2) occlusion of the suprahepatic segment of the IVC.43–49 PTA is ideally suited for treating focal stenoses, and the high success rate can obviate the need for more invasive surgery. Although more lengthy stenoses eventually may require placement of a venous stent, PTA alone can be used as the initial treatment. In cases of recurrent stenosis, obstruction, or web(s), PTA can be repeated as often as is needed (and practical) with minimal risk, short-term hospitalization, and acceptable cost. In addition, PTA does not preclude future use of more invasive therapy such as thrombolysis, vascular stenting, surgical resection of the obstruction, or portosystemic shunting.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree