Introduction
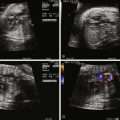
We begin our examination of the normal fetal circulation with a description of the anatomical pathways involved ( Figure 1-1 ).

Oxygenated blood leaves the placenta via the umbilical vein (UV). From the UV, between 20% and 50% of the blood flows into the ductus venosus (DV), which joins the inferior vena cava (IVC) shortly before it enters the floor of the right atrium (RA). The rest of the UV blood perfuses the liver, and then rejoins the IVC circulation via the hepatic veins. Blood within the IVC that originated from the DV is mainly streamed preferentially through the foramen ovale (FO) into the left atrium (LA), through the mitral valve (MV) into the left ventricle (LV), and then out through the aortic valve (AoV) and into the ascending aorta (AAo). This blood then flows across the aortic arch, where it provides relatively oxygenated blood to the head, myocardium, and upper body via the coronary, carotid, and subclavian arteries, with a small portion continuing on via the aortic isthmus to the descending aorta (DAo).
Deoxygenated blood from the superior vena cava (SVC), together with the majority of non–DV-originating blood in the IVC flows into the RA, through the tricuspid valve (TV) into the right ventricle (RV) and out through the pulmonic valve (PV) into the pulmonary artery (PA). From the PA, approximately 20% of the blood flows to the lungs, with the remainder flowing through the ductus arteriosus (DA) to join the DAo, where it makes up the majority of the flow. The blood flowing through the DAo supplies the internal organs and the lower body, as well as the two umbilical arteries that return blood to the placental circulation. Thus, the fetal circulation is essentially a parallel circulation with three circulatory “shunts”: the DV, the FO, and the DA. This circulatory design has a targeted goal—the brain, coronary circulation, and upper body are essentially supplied with relatively oxygenated blood via the LV, whereas the lower body receives mainly deoxygenated blood via the RV.
The majority of foundational research into the fetal circulation has been carried out on fetal sheep, which have the advantage of being large mammals, yet with a gestational duration about half the length of humans. More recent research based on ultrasonographic and Doppler studies has highlighted important differences between humans and sheep. This is perhaps not surprising because sheep fetuses have two UVs, a faster growth rate, a higher body temperature, a lower hemoglobin, a smaller brain, a differently positioned liver, and a longer intrathoracic IVC.
Ductus Venosus, Hepatic Circulation, and Inferior Vena Cava
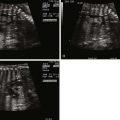
The DV is a small vessel that has been variously described as being shaped like a trumpet or an hourglass. It connects the UV to the IVC as it enters the RA, at the confluence with the hepatic veins ( Figure 1-2 ). Early animal studies indicated that 50% of UV blood flow was channeled into the DV, with the amount of shunt through the DV proportional to the UV flow, implying a significant physiological role for this pathway. However, more recent studies of human fetuses using noninvasive ultrasonographic techniques have shown that the amount shunted through the DV is less and, moreover, that there is a decrease in shunting throughout gestation (i.e., more UV blood traversing the liver with later gestation). Kiserud and coworkers demonstrated that the percentage of blood shunted through the DV decreases from approximately 30% at 18 to 19 weeks’ gestation to approximately 20% at week 30, although with wide variations between subjects. Bellotti and colleagues found that the percentage shunted was approximately 40% at 20 weeks, decreasing to approximately 15% at term. Work based on mathematical impedance modeling of the hepatic venous network suggests that the shunt decreases from 50% at 20 weeks to 20% at term. Interestingly, the data suggesting a shunt of 50% from the original seminal study of Rudolph and Heymann in 1967 do not appear to be controlled for gestational age. Thus, there may be less conflict between the animal and the human data than has been suggested. As a greater percentage of UV return is directed through the liver with later gestation, it raises the speculation of the liver playing an important role in third-trimester fetal maturation and growth through the release of proteins and mediators. The role of the liver as “gate-keeper” to placental venous return in the growing fetus is a fascinating one, and still poorly understood.
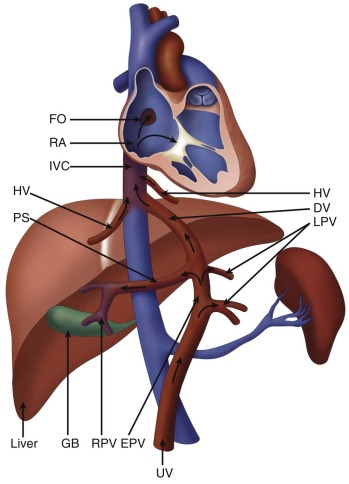
Within the IVC entry site at the floor of the RA, the column of blood originating from the DV is preferentially streamed across the FO into the LA, and the remainder enters the RA and crosses the TV. The mechanism by which this occurs is likely related to the complex geometry of the vessels as they enter the RA floor; the phenomenon can be demonstrated on Doppler color flow mapping. In sheep, there are valvelike structures at the opening of the DV and left hepatic vein that may physically direct the different flows from within the IVC. However, these structures do not appear to exist in the same way in the human fetus. Kiserud and Acharya suggest that the rapid increase in velocity of the blood within the DV, caused by the pressure gradient, means that the blood column originating from the DV has the highest kinetic energy; thus, it is this blood that opens the FO valve and enters the LA.
A related controversy concerns the presence of a sphincter mechanism within the DV by which flow may be increased or decreased. It has been demonstrated, in both animal and human models, that the flow through the DV is increased in certain conditions such as hypovolemia and hypoxemia. Some studies have favored the presence of a discrete sphincter mechanism that controls the caliber of the DV, whereas others propose that the entire vessel is tonically controlled by neurohumoral mechanisms. Alternatively, a drop in resistance to flow through a relaxation of the portal vascular system may direct blood away from the DV. This notion is supported by the finding of a greater degree of smooth muscle in the walls of the fetal portal venous system than in the DV.
Blood from the left hepatic vein is also shunted preferentially through the FO, owing to the position of its entry into the IVC just under the eustachian valve. In fact, the liver, despite its high metabolic activity in the fetus, extracts relatively little oxygen (10–15% ), such that hepatic venous blood is fairly well oxygenated and, thus, potentially contributes to the highly oxygenated blood-streaming phenomenon within the fetal heart.
Foramen Ovale
In the postnatal human infant, the FO is commonly thought of as being a connection between the two atria, causing shunts from one side to the other. It has also been described as such in the fetus. However, it is contended that in the fetus, the anatomical and functional arrangement is different. The FO flap and the crista dividens of the interatrial septum act as a “valve,” directing the stream of blood from the IVC, which enters essentially between the two atria from below. The stream of blood is divided due to position, direction, and velocity, with DV and left hepatic venous blood directed to the left atrium, while abdominal IVC blood is directed to the RA. Changes in pressure on either side will change the balance of flow, and this can have far-reaching consequences for the development of the fetal heart. For example, in aortic stenosis, left atrial pressure is elevated thereby increasing shunting of blood to the RA, which by neglecting the LA, may eventually leading to left-sided hypoplasia, although the causal chain of events is very much controversial. Experimental models have shown that normal flow distributions within the developing heart may be critical for normal cardiac morphogenesis.
Ductus Arteriosus
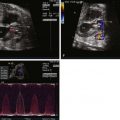
The DA is a large vessel with muscular walls, which connects the pulmonary trunk and aorta. The systolic flow within the DA has the highest velocity of all the fetal cardiovascular system, and the velocity increases with increasing gestational age. The human DA shunts an estimated 78% of the right ventricular output, or 46% of the combined cardiac output (CCO), away from the lungs to join the DAo and perfuse the lower body. These figures are slightly lower than in sheep models, which suggest that the DA carries 88% of the right ventricular output and 58% of the CCO. The patency of the DA depends on levels of circulating prostaglandin E 2 (PGE 2 ), but the flow through the DA is dependent on the resistance of the pulmonary vasculature. The pulmonary vasculature undergoes changes during the third trimester of gestation such that increases in partial pressure of oxygen (PO 2 ) cause resistance to decrease and, therefore, flow through the DA to change accordingly. This mimics the physiological processes that take place after birth with the onset of breathing and can theoretically be used as an in utero test for fetal pulmonary vascular development such as in conditions of congenital heart disease or pulmonary hypoplasia.
The sensitivity of the DA to PGE 2 in utero has clinical significance, because maternal administration of PGE 2 inhibitors such as indomethacin can cause the DA to close with catastrophic consequences. The response to indomethacin is thought to be potentiated by stress, and intraoperative echocardiography demonstrates that indomethacin used in fetal surgery induces more potent constriction of the DA. Interestingly, there seems to be some “physiological” constriction of the DA as gestation proceeds toward term, which may explain the increased velocity that is seen in the DA relative to the PA. Because the lungs represent a major site of PGE 2 metabolism, it would seem plausible that this constriction of the DA is due to increased prostaglandin degradation because pulmonary perfusion increases toward the end of gestation.
Aortic Isthmus
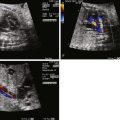
The isthmus of the aorta (the section of the aortic arch between the take-off of the left subclavian artery and the insertion of the DA) represents a watershed region between the aortic arch, which transmits relatively well oxygenated blood to the head and upper body, and the DA, which transmits relatively deoxygenated blood to the lower body. The isthmus may also represent a functional division between these two arterial circuits, because noradrenaline and acetylcholine injected into either side of the isthmus in the fetal lamb can be demonstrated to affect only that side for at least a few heartbeats. Animal studies have shown that, under physiological conditions, only 10% to 15% of the CCO is transmitted through the isthmus because the majority of blood in the ascending aorta is distributed to the myocardium, head, and upper limbs via the coronary, carotid, and subclavian arteries. One of the most important hemodynamic factors influencing the direction of flow through the isthmus is the relative resistances of the cerebral and placental circulations. If the placental resistance (which is normally very low) increases sufficiently, the two circuits (upper and lower body) can be separated, with blood ejected from the LV perfusing the heart and upper body only, with negligible forward flow (because the placenta is no longer the site of lowest vascular resistance). Meanwhile, the RV perfuses the lower body exclusively. As placental resistance progressively increases, retrograde flow can be detected in the isthmus. Indeed, the isthmus represents an example of the plasticity of the fetal circulation to adapt to varying circumstances. For example, as in cases of reduced left ventricular output, DA blood flows retrograde through the isthmus to supply the AAo and aortic arch.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree