David J. Lomas, Lorenzo Mannelli
The Liver and Spleen
Liver
Anatomy1–4
The liver has a dome-shaped superior surface following the diaphragm contours extending anteriorly to the inferior edge of the liver. The major surface landmark is a sagittal groove containing the ligamentum teres (formerly umbilical vein), within the falciform ligament. The main feature of the inferior or visceral surface is the porta hepatis or hilum, a central depression conveying the portal vein, hepatic artery and common bile duct. The gallbladder fossa is positioned anterior to the hilum with the quadrate surface to the left. Posteriorly the caudate lobe separates the porta from the inferior vena cava (IVC). Several shallow surface impressions relate to adjacent organs, such as the right kidney. Normal liver volume, derived from postmortem studies of liver weight, ranges from 1 to 2.5 kg, and varies with gender, age and body mass. Liver weight is maximal in the fifth and sixth decades and subsequently declines rapidly. Riedel’s lobe is an extension of the tip of the right lobe inferior to the costal margin based on clinical palpation; the term is misleading as it does not represent an anatomically discrete lobe or segment and is now considered part of the normal spectrum of liver shape and size (Fig. 31-1).
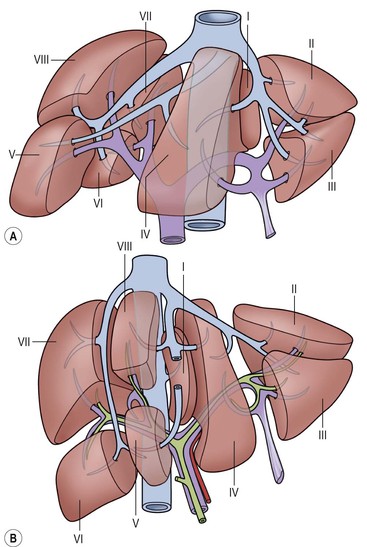
Subdivisions1 (Fig. 31-2)
Studies of the vasculature demonstrate an internal craniocaudal principal plane (dividing the liver into left and right) not usually visualised on imaging techniques. The principal plane is defined by three key landmarks: the IVC groove, the middle hepatic vein and the gallbladder fossa. The liver is further subdivided into Couinaud segments based on the vascular supply. The caudate lobe or segment I has an autonomous blood supply from both left and right branches of the portal vein and hepatic artery along with independent venous drainage directly into the IVC.
Liver parenchyma has a lobular structure each comprising a central draining vein surrounded by sinusoids bounded peripherally by portal tracts, each a ‘triad’ of adjacent branches of the bile duct, portal vein and hepatic artery. At cellular level the liver is mainly composed of hepatocytes, stellate cells, and Kupffer cells, part of the reticulo-endothelial system. The liver receives approximately two-thirds of its blood supply from the portal vein and one-third from the hepatic artery. Blood drains via the hepatic veins to the IVC. During a meal, mesenteric blood flow volumes may double, increasing portal vein flow volumes correspondingly. The pressure difference between measurements in the wedged (occluded) hepatic vein and the IVC (the corrected sinusoidal pressure) is normally between 4 and 8 mmHg.
Lobar Agenesis/Atrophy3 (Figs. 31-3 and 31-4)
Right and left lobe agenesis has been reported but is controversial: the absence of supplying vasculature or dilated bile ducts is said to permit the diagnosis of true agenesis rather than early atrophy. Surgical hemihepatectomy or disease-related atrophy is more common. In normal livers compensatory hypertrophy of the remaining lobe often occurs with corresponding displacement of the gallbladder.
Vascular Anatomy Variation5,6 (Fig. 31-5)
The common hepatic artery is one of the three major branches of the coeliac axis. After giving off the gastroduodenal artery, the main hepatic artery continues and divides into the right and left hepatic arteries. Variations of the hepatic arterial supply are important for radiologists and hepatic surgeons. The portal vein divides into right and left branches and variations are infrequent, although early branches arising from the main trunk or close to the main division may create problems during liver resection. Three major hepatic veins drain into the IVC in 70% of cases, but in the remaining 30% accessory veins occur (19% having two left hepatic veins, 8% two right hepatic veins and 2% two middle hepatic veins). Absence of the IVC is rare and associated with complete situs inversus but may occur with partial situs and a right-sided liver. In this circumstance the hepatic veins drain direct to one of the cardiac atria with the azygos vein replacing the IVC, passing posterior to the diaphragmatic crura into the chest. More commonly, aberrant gastric venous drainage of the posterior aspect of segment IV may occur and has been correlated with focal fat variation. Accurate definition of the vascular and biliary anatomy is particularly important before live donor liver transplantation.
Liver Imaging Techniques
Plain Radiography
Plain radiographs are now rarely useful for liver evaluation, but may demonstrate gross hepatomegaly and hepatic calcification. The complex shape of the liver, limited soft-tissue contrast and projection acquisition of plain radiographs makes reliable identification of the liver boundaries difficult.
Ultrasound
Technique
Abdominal ultrasound (US) is routinely used with phased array transducers operating between 3 and 5 MHz, and Doppler capability, both spectral, colour and harmonic, is an integral part of the examination of the liver, allowing demonstration of hepatic blood flow and unequivocal bile duct identification. Contrast-enhanced US9 is variably used to add an arterial and portal phase study comparable with CT and MRI.
Normal7,8 (Fig. 31-6)
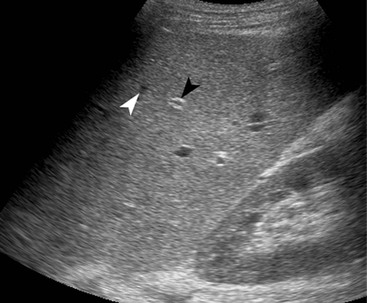
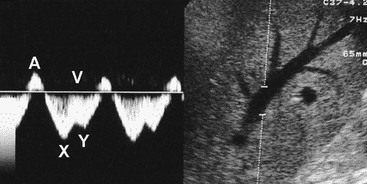
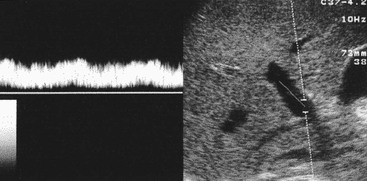
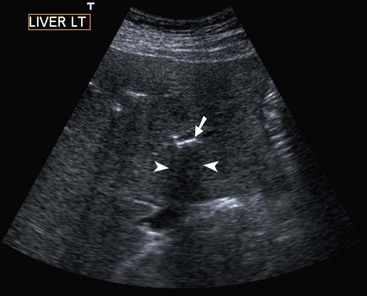
Normal liver parenchyma echo texture is homogeneous and slightly more reflective than adjacent renal cortex. Portal vein branches radiate from the hilum and have increased wall reflectivity. Hepatic veins converge on the IVC and right atrium and have walls indistinguishable from the adjacent parenchyma. At Doppler examination the normal hepatic vein waveform reflects the transmitted right heart pressure changes with transient flow reversal flow during the cardiac cycle (Fig. 31-7). The portal vein waveform is normally continuous antegrade (mean peak velocity approximately 15–25 cm/s) and may vary slightly with respiration and the cardiac cycle (Fig. 31-8).
Computed Tomography
Technique9–11 (Fig. 31-9)
Current volumetric CT systems allow complete isotropic data acquisition of the upper abdomen in a few seconds and choice of section thickness post acquisition. Unenhanced imaging remains valuable for assessing diffuse hepatic changes, such as fat infiltration and iron deposition, and for evaluating focal changes, in particular subtle calcification and haemorrhage. Dual energy systems may in future remove the need for a separate unenhanced acquisition and provide new characterisation methods (Fig. 31-10). Multiphase contrast-enhanced imaging following IV administration of water-soluble iodinated contrast medium is routinely used for detection and characterisation of focal lesions. Many solid liver lesions have a predominantly arterial blood supply, whereas the liver parenchyma receives 75–80% of its blood supply via the portal vein. During contrast enhancement early and late arterial phase studies. Along with portal and delayed phase imaging, may be obtained. Optimising protocols and phase timing to maximise lesion-to-liver contrast varies with individual CT system but the minimum requirement for liver imaging is typically a relatively late arterial phase (e.g. centred 18 s post contrast medium arrival in the abdominal aorta) and a portal venous phase. Delayed CT imaging is used in selected cases, e.g. haemangiomas, and cholangiocarcinoma. CT arteriography (CTA) and CT arterioportography (CTAP) using direct hepatic artery injection during CT examination and Lipiodol CT are now rarely used.
Normal
Liver parenchyma is homogeneous with attenuation values of 54–60 Hounsfield units (HU), usually 8–10 HU greater than the spleen. Vascular structures can be identified by their location on the unenhanced images and confirmed by enhancement with IV contrast medium. The peripheral intrahepatic biliary tree is not normally visualised, although the main right and left hepatic ducts and the common hepatic and bile ducts are normally demonstrated.
Magnetic Resonance Imaging
Techniques12–14 (Fig. 31-11)
MRI has a wider range of contrast mechanisms than other imaging techniques and is increasingly used for lesion detection and characterisation. Biliary tract anatomy and hepatic vascular patency can be assessed during the same examination. A wide range of protocols is available because of the numerous combinations of field strength, pulse sequence implementation and interdependent sequence parameters, all of which can influence image quality. Multi-coil surface arrays are essential and most studies are mainly breath-hold examinations as rapid MRI sequences can rival CT, although they may have compromised contrast performance that may limit lesion detection sensitivity. This is traded off with improved anatomical definition of extrahepatic structures. A typical MRI protocol includes breath-hold T2- and T1-weighted (T2w and T1w) imaging, and chemical shift imaging for hepatic steatosis detection. High-quality T2w imaging can be obtained with respiratory-triggered multi-shot RARE sequences and pre- and multiphase post-gadolinium imaging using rapid breath-hold 3D T1w volume imaging is now routine. Diffusion-weighted imaging (DWI) is increasingly used to improve liver lesion detection. MR-based quantification has been developed for the measurement of hepatic steatosis, iron and fibrosis using chemical shift imaging, T2 and T2* relaxometry and elastography. These techniques are undergoing standardisation and validation but are starting to enter routine clinical practice.
Intravenous Contrast Agents12,15–17 (Fig. 31-12)
Both non-specific intravenous gadolinium agents and liver-specific agents are in routine clinical use. Gadolinium-based agents that equilibrate rapidly with extracellular fluid include Gd-DTPA and GD-DOTA, as well as the more recent non-ionic agents gadodiamide, gadobutrol and gadoteridol. These agents provide enhancement on T1w images in a similar fashion to iodinated contrast media at CT examination. Breath-hold 3D T1w sequences allow the acquisition of multiphasic (arterial, portal, delayed) examinations as for CT. The enhancement characteristics for many focal lesions are, not surprisingly, similar to those for CT.
Hepatobiliary specific agents have been developed which target either the reticulo-endothelial system (RES) or hepatocytes. Iron oxide particles possess superparamagnetic properties that create susceptibility-induced dephasing of protons, thereby shortening T2. A range of ultra-small paramagnetic iron oxide (USPIO) agents have been developed with varying sizes and properties targeting mainly the reticulo-endothelial cells but also capable of functioning as blood pool agents for vascular studies. The availability of the iron agents varies across the world and in some regions they have been withdrawn probably due to declining utilisation.
Mn-DPDP (mangafodipir trisodium), Gd-BOPTA (gadobenate dimeglumine) and most recently Gd-EOB-DTPA (gadoxetate) are all hepatocyte-specific paramagnetic agents which accumulate in hepatocytes followed by biliary excretion. They cause enhancement of the normal liver parenchyma and biliary tree on T1w imaging and indicate the presence of hepatocyte function. Mn-DPDP is no longer available but the other agents have been used for increasing the sensitivity of liver lesion detection, lesion characterisation and the study of the biliary tract.
Normal
The intensity of normal liver parenchyma is the same as, or slightly higher than, that of adjacent muscle. This holds for all sequence combinations except for inversion recovery techniques with inversion times that completely null liver signal. The appearance of vessels varies widely on MRI depending on pulse sequence, artefact suppression techniques and contrast media. In particular, intravascular signal on conventional spin-echo sequences may occur normally and should not be interpreted as thrombus without confirmation using a reliable time-of-flight or contrast-enhanced technique. In routine practice liver–spleen differences are helpful as a simple guide to effective intrinsic T1 and T2 weighting. In general the spleen should be lower signal than the liver on effectively weighted T1w images and higher signal than the liver on T2w images.
Scintigraphy18
Radionuclide imaging of the liver for lesion characterisation has been largely superseded by the other techniques but is employed when they are unavailable or inappropriate. Studies typically use 99mTc-sulphur colloid or albumin colloid, which target the reticulo-endothelial system. Positron emission tomography (PET) combined with CT is increasingly used in oncology but, where FDG based, is rarely used for primary liver disease owing to the normal high liver uptake. This position may change as more selective radionuclides become available.
Technique
Liver/spleen imaging is usually performed following injection of a colloid agent such as 99mTc-sulphur colloid, injected intravenously. The majority of the colloid is taken up by the Kupffer cells in the liver and 5–10% is taken up by the spleen. A small portion is also absorbed by the bone marrow. Sulphur colloid is cleared rapidly from the bloodstream (t1/2 = 2 min) and in patients with normal liver function imaging may begin 5–10 min after injection but in those with compromised hepatic function and/or portal hypertension, optimal concentration of the sulphur colloid will take longer and imaging can be delayed to take account of this.
Gamma camera images are obtained in multiple projections and liver/spleen angiographic and blood flow phases can also be obtained at the start of a study by acquiring rapid sequential images during the first 30–60 seconds. Single-photon emission computed tomography (SPECT) imaging can be employed to evaluate suspicious areas for focal or diffuse space-occupying disease. PET and PET-CT imaging can provide both projection and tomographic images using a range of cyclotron-generated radionuclides with varying half-lives.
Normal
The evaluation of a sulphur colloid scintigram involves an assessment of liver size, shape, distribution of the radiopharmaceutical within the spleen, liver and bone marrow, and the homogeneity of uptake within the liver and spleen. Peripheral indentations on the liver are normally produced by the lateral rib margins, xiphoid process, gallbladder, right kidney and heart. The hepatic veins make a triangular impression on the superior, central margin of the liver, and the porta hepatis makes an impression on the inferomedial segment of the right lobe.
Angiography5,18,19
Catheter-based intravascular angiography is dealt with in a separate chapter and its use in liver disease summarised here. Arteriography is best performed by selective catheterisation, and the arterial and parenchymal phases of the study are usually of most diagnostic value. The hepatic veins are seen routinely on digital subtraction angiography but the portal vein is not normally visualised on an arteriogram unless there has been flow reversal or an arterioportal shunt is present.
Portal venography is performed either directly or indirectly by portal vein or splenic pulp puncture. Indirect portography (arterioportography) is less hazardous than direct methods and combines an arterial study. Direct methods (including percutaneous splenic, transhepatic and transjugular approaches) are now used only when therapeutic procedures (e.g. transjugular intrahepatic portosystemic shunt (TIPSS)) or sampling techniques (e.g. direct portal venous pressure measurement) are being employed.
Hepatic venography is performed following retrograde catherisation usually via the femoral or jugular veins. Filling of the small hepatic venous radicles is assisted if the patient performs a Valsalva manoeuvre. Hepatic venous wedge pressure measurement is performed by impacting an end-hole catheter in a small branch of an hepatic vein.
Diffuse Disease
Diffuse hepatic diseases are more difficult to detect than focal lesions as their effect on normal liver architecture may be minimal. Diagnoses are often made on the basis of clinical features with histological confirmation. Imaging can help assess extent and severity of diffuse disease by demonstrating liver abnormalities and sequelae such as portal hypertension changes. Recently MR techniques have been developed that provide quantification of hepatic steatosis, iron and fibrosis.
Benign Diffuse Disease
Hepatic Steatosis20–22
Diffuse steatosis is an increasingly common finding reflecting increased triglyceride loading of hepatocytes. It has a wide range of causes, including acute and chronic alcohol abuse, obesity, diabetes mellitus, insulin resistance, cystic fibrosis, malnourishment, total parenteral nutrition, tetracyclines, steroids and ileal bypass. Linkage to metabolic syndrome and cardiovascular disease make this formerly ignored condition the subject of much research interest. Focal fat variation is also common and discussed later.
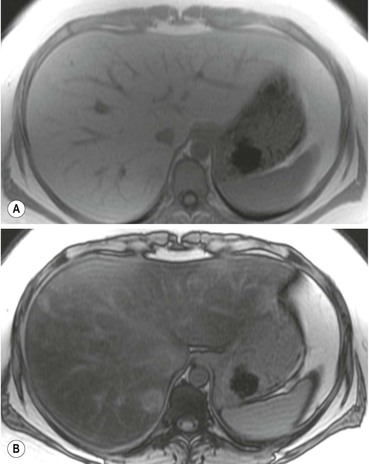
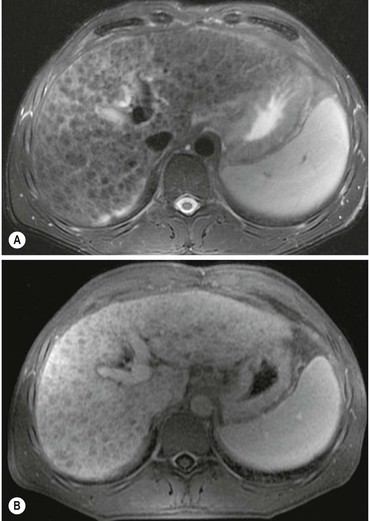
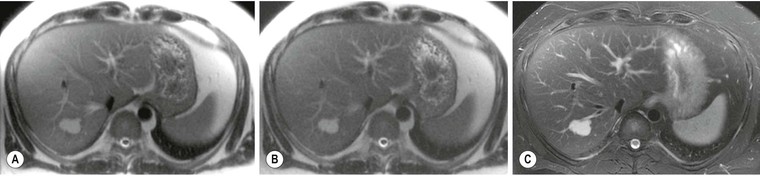
US detects hepatic steatosis through increased parenchymal reflectivity, which obscures the portal vein margins (Fig. 31-13). Although this finding can be virtually diagnostic, further imaging may be required as fibrosis can also cause increased reflectivity. CT can demonstrate and quantify diffuse hepatic steatosis as the attenuation decreases by approximately 1.6 HU per mg of triglyceride increase per gram of liver substance. Confounding changes such as fibrosis, drug treatment and conditions such as haemochromatosis make this unreliable. The liver architecture is preserved, especially the vascular pattern and the liver enhances normally following IV contrast medium. With increasing fat infiltration the liver attenuation decreases, reversing, in turn, the normal liver–spleen difference and liver–blood difference (Fig. 31-14). MRI is the most sensitive and specific technique for demonstrating hepatic steatosis. Dixon-based (Fig. 31-11), ‘chemical shift’ or ‘in- and out-of-phase’ imaging (Fig. 31-15) allow both an accurate diagnosis and, with appropriate T2 and other corrections, accurate quantification. MRI is also the most accurate test for diagnosis of focal fat variation.
Cirrhosis23–29
Cirrhosis is the end stage of a wide variety of hepatic disease processes that cause hepatocellular inflammation and necrosis leading to hepatic fibrosis and nodular regeneration. Early changes may be detectable only on histological examination. As cirrhosis progresses, widespread fibrosis and nodular regeneration develop, along with macroscopic changes of liver morphology which can be detected on imaging. Liver stiffness also increases but the commonest anatomical finding in advanced cirrhosis is atrophy of the posterior segments (VI, VII) of the right lobe. Hypertrophy of the caudate (I) lobe and of the lateral segments of the left lobe (II, III) is frequently seen. In primary sclerosing cholangitis caudate lobe hypertrophy is found in virtually all cases and the lateral segments of the left lobe (II, III) occasionally atrophy. The cause of these changes is uncertain but thought to be blood flow related. Hepatic and portal system dynamics may alter radically in cirrhosis, with both increased overall hepatic blood flow (through intrahepatic arteriovenous shunts) and decreased hepatic blood flow (resulting from increased intrahepatic vascular resistance) recognised in advanced disease.
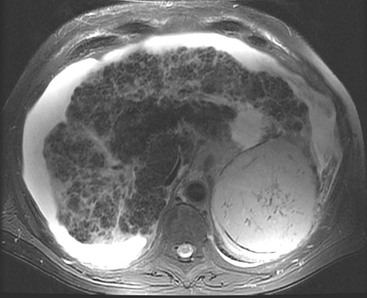
US can demonstrate the nodularity of the liver margin in advanced cirrhosis, particularly when ascites is present and when using high-frequency transducers. Pure hepatic fibrosis increases reflectivity, resulting in loss of the margins of the portal vein branches, but is thought not to alter attenuation, a feature in the past used to discriminate steatosis from fibrosis but in practice the two often coexist making separation difficult. The liver texture becomes coarser or more heterogeneous as cirrhosis progresses, but this is difficult to quantify and subjective. As the liver atrophies in end-stage cirrhosis, the hepatic veins may become attenuated and difficult to visualise. Doppler US examination may reveal other non-specific features of cirrhosis: damping of the normal right heart waveforms in the hepatic veins, reduced main portal vein blood flow (<10 cm/s mean peak) or hepatofugal flow. Occasionally increased flow in a large recanalised para-umbilical vein will ‘steal’ blood from the right portal vein branch, leading to reversed flow in the right portal vein but normal hepatopetal flow in the main and left portal veins. Hepatic arterial flow is usually increased in advanced cirrhosis as the portal contribution to hepatocyte perfusion decreases. This results in enlargement of the hepatic arterial system, which can be mistaken for enlarged bile ducts on US unless Doppler techniques are used to identify the vessels. Over the last decade several forms of ultrasound elastography have been developed that evaluate liver stiffness. These vary from a 1D non-imaging method ‘transient’ elastography to a pulsed shear wave method combined with 2D imaging ‘acoustic radiation force imaging’. Several of these methods provide absolute quantification of liver stiffness and large trials suggest that these techniques may have a role in the detection and quantification of liver fibrosis although their exact role in patient management is not yet clear.
CT (Fig. 31-16) is insensitive to early fibrosis changes but demonstrates the nodular margin and lobar atrophy/hypertrophy changes of advanced disease. On unenhanced examinations regenerative areas have relatively normal attenuation but advanced fibrosis lowers attenuation, whereas the accumulation of iron in hepatocytes increases it. These features frequently coexist in many forms of cirrhosis, resulting in parenchymal heterogeneity both before and after enhancement with IV contrast medium. Portal phase imaging can be helpful in assessing portal vein patency, although flow volume and direction cannot be determined.
MRI is also insensitive to early fibrosis changes and there are no specific changes of parenchymal signal intensity on T1w or T2w imaging, although parenchymal heterogeneity (Fig. 31-17) may occur on T2w and delayed post-gadolinium T1w imaging, but is difficult to quantify. MRI delineates the morphological changes of advanced cirrhosis but can also provide non-invasive assessment of portal vein patency along with flow direction and bulk flow volume estimation when other techniques have proved unhelpful. Studies using DWI and 31P spectroscopy have given mixed results for trying to grade fibrosis. MR elastography is a relatively new technique quantifying liver stiffness in a similar fashion to US methods. This technique appears promising for detecting the relatively early stages of hepatic fibrosis and further research is ongoing.
Colloid scintigraphy is rarely used but in established cirrhosis demonstrates reduced, heterogeneous hepatic uptake and increased extrahepatic uptake. In advanced disease morphological changes may be detected. Angiography may be used to assess vascular complications such as variceal bleeding and portal hypertensive changes. Hepatic arteriography in cirrhotic liver demonstrates increased tortuosity of intrahepatic branches, so-called ‘corkscrew vessels’, which reflect lobar shrinkage.
Viral Hepatitis30
Viral hepatitis, including hepatitis B and hepatitis C, remains a major public health concern as it may lead to liver failure and primary liver cancer, often detected late. Diagnosis and monitoring based on serological tests and imaging is relatively non-specific. In acute hepatitis, imaging excludes obstructive causes of jaundice. In chronic hepatitis with cirrhosis, imaging helps monitor disease progression, development of portal venous hypertension and complications such as hepatocellular carcinoma (HCC).
On US examination non-specific decreased reflectivity occurs in acute viral hepatitis, although the majority of cases have normal parenchyma. Gallbladder wall thickening is a common non-specific finding in acute hepatitis. On colloid scintigraphy the appearance of hepatitis is similar to the early stages of cirrhosis, with uneven and reduced uptake. In chronic hepatitis CT, MRI and angiography are of limited value until cirrhotic changes develop.
Haemochromatosis and Iron Overload31,32
Haemochromatosis and multiple transfusions may both result in iron deposition in the liver. Inherited genetic haemochromatosis causes hepatocyte iron accumulation (leading to subsequent cirrhosis) and iron accumulation in other organs, including myocardium, skin and endocrine glands. Affected individuals have an increased risk of developing malignancy in general and of hepatocellular carcinoma in particular. Initially the hepatic iron deposition is diffuse but the development of cirrhosis and regenerative changes often results in uneven distribution. By comparison hepatic iron overload from multiple transfusions (haemosiderosis) results in iron accumulation in the reticulo-endothelial system (Kupffer cells) in the liver, bone marrow and spleen. There is less risk of liver damage and the pattern of organ involvement can aid diagnosis.
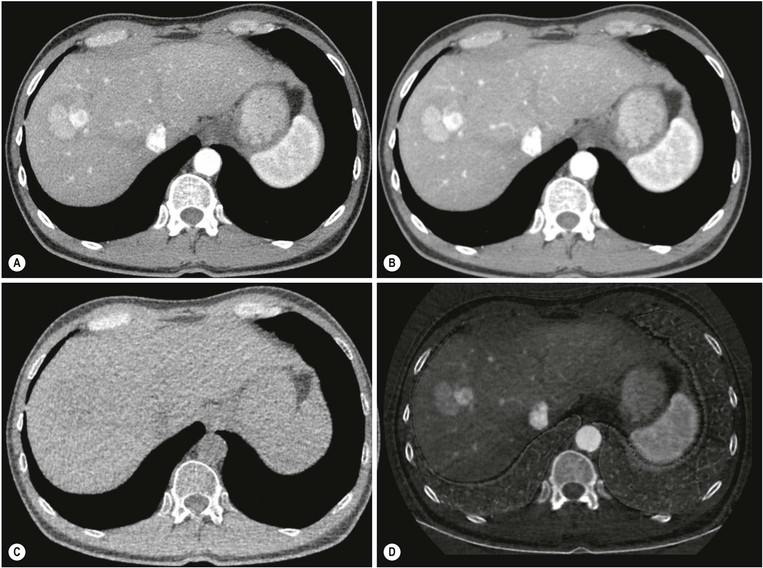
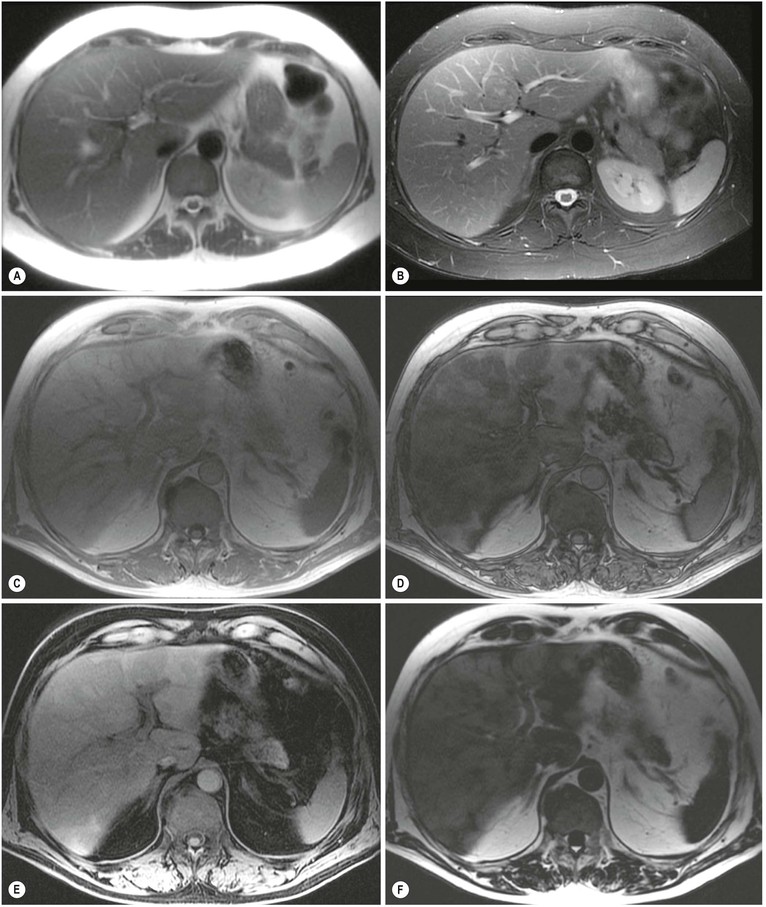
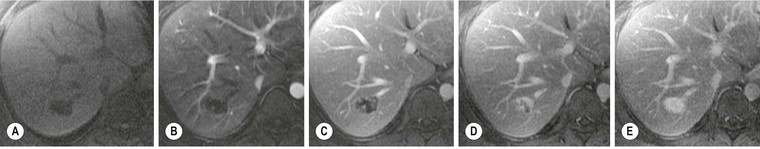
MRI (Figs. 31-18 and 31-19) is the most specific imaging technique, as intracellular iron exerts a local susceptibility effect, reducing parenchymal T2 and T2*. This effect is most sensitively detected by T2*w gradient-echo imaging although with significant accumulation the effect is easily seen on T2w spin-echo images, and when severe will affect T1w images. The liver signal is abnormally reduced (to less than that of adjacent muscle). Several studies have demonstrated that hepatic iron concentration correlates strongly with both T2* and T2 value, permitting accurate quantification. Abnormally reduced signal on T2w imaging is the main feature in other affected organs such as spleen and pancreas. Unenhanced CT demonstrates hepatic iron deposition through an increase in HU value (>75 HU) (Fig. 31-20) but this also occurs in amiodarone treatment and previous Thorotrast exposure. The changes are unreliable because of the confounding effect of steatosis. US may demonstrate increased parenchymal reflectivity but there are no specific features that characterise iron deposition.
Wilson’s Disease
Wilson’s disease is an autosomal recessive disorder in which copper is deposited in the liver, as cornea and lenticular nucleus of the brain. Copper is hepatotoxic and triggers inflammation that progresses to cirrhosis. Imaging demonstrates the generalised cirrhotic changes but the underlying cause is rarely evident. Copper accumulation rarely causes a detectable increase in hepatic attenuation on CT, and there is often coexistent steatosis counteracting the effect. On MRI there may be a subtle increased signal on T1w with a decrease on T2w images. There are no specific features on US studies.
Malignant Diffuse Disease
Occasionally the liver is diffusely involved by malignancy, usually metastatic disease, e.g. breast carcinoma, which may give a diffusely increased echo-reflective and heterogeneous appearance on US. Some primary hepatic tumours, including hepatocellular carcinoma, may present with non-specific diffuse infiltrative changes. Lymphoma and leukaemia may also cause diffuse hepatic infiltration demonstrated by US as non-specific reduced echo reflectivity. In all these situations the diagnosis is difficult to make although subtle heterogeneity that cannot be attributed to cirrhosis or fat infiltration is usually evident on most imaging techniques. The presence of other abnormalities (e.g. vascular thrombosis with HCC) may be helpful, but in the appropriate clinical context biopsy may be required to detect diffuse malignant involvement.
Focal Disease
Calcification33
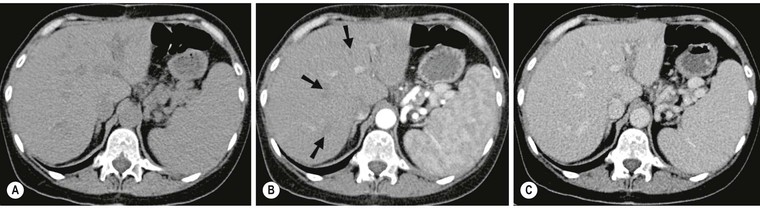
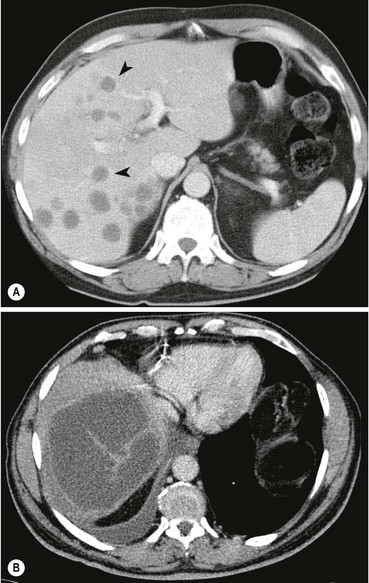
Benign parenchymal calcification may occur following focal insults such as tuberculosis, Pneumocystis infection, sarcoidosis, pyogenic abscess and parenchymal haematoma. The calcification is well demarcated and surrounded by otherwise normal parenchyma. In Pneumocystis carinii infection widespread focal calcification may occur. Focal calcification also occurs within benign lesions (giant haemangioma) and malignant lesions, particularly mucin-secreting adenocarcinoma of the colon, where it is often relatively ill defined. Primary liver tumours such as hepatoblastoma and fibrolamellar hepatoma may also contain foci of calcification.
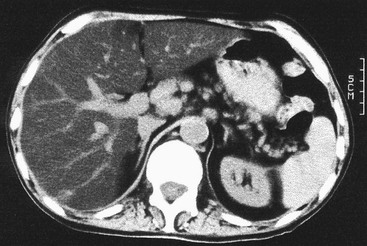
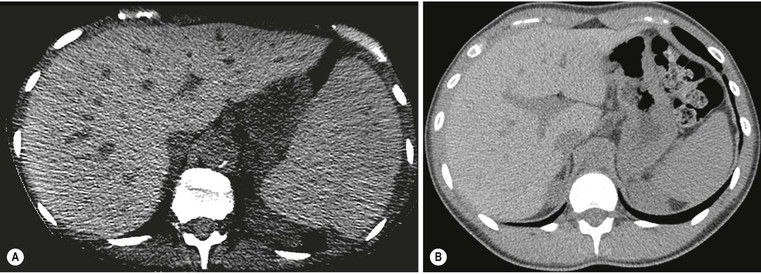
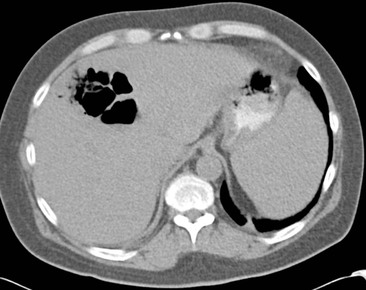
Plain radiographs demonstrate gross calcification, but unenhanced CT is more sensitive and detects subtle calcification, e.g. metastases (Fig. 31-21). US clearly demonstrates focal calcification, with increased reflectivity and a posterior acoustic corridor, but this feature alone does not always allow distinction from focal gas. Scintigraphy and MRI are insensitive to calcification.
Pneumobilia
Gas in the biliary tract may occur as a result of a sphincterotomy, or Roux loop procedure allowing reflux of intestinal gas into the biliary tree. It can be identified by the linear distribution radiating from the hilum and gravity dependence with air predominantly in the nondependent parts of the biliary tree.
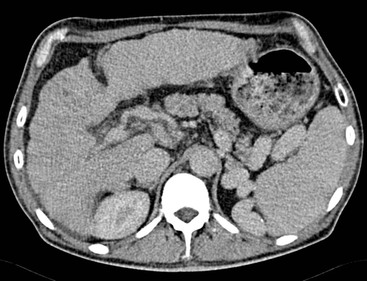
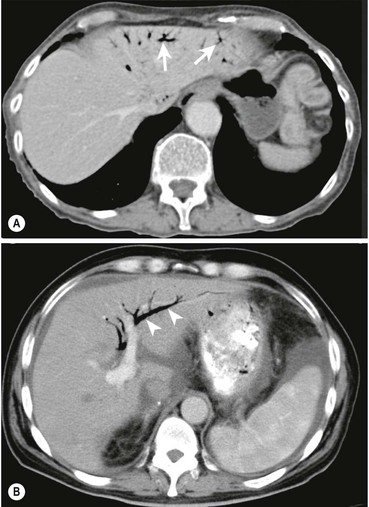
Both US and CT (Figs. 31-22 and 31-23) demonstrate clearly pneumobilia and its distribution. On US the ducts are increased echo-reflectivity linear structures that may be differentiated from calcification by the pattern and movement of the gas related to respiration, bowel peristalsis or patient position. CT is extremely sensitive to the presence of gas, which is easily demonstrated and localised.
Portal Vein Gas34
Portal vein gas is always abnormal and occurs when intestinal permeability increases and/or there is an increase in intestinal luminal pressure. These conditions are fulfilled in neonatal necrotising enterocolitis but also in adults with gastric emphysema, intestinal obstructions, infections and Crohn’s disease. It has also been described in blunt abdominal trauma, invasive abdominal malignancies (colon carcinoma, ovarian carcinoma), duodenal perforation at ERCP and in patients with colitis following a barium enema. The significance and outcome largely relates to the underlying aetiology. The gas typically radiates out from the hilum with less marked gravity dependence than pneumobilia and a more peripheral distribution (Fig. 31-22).
US sensitively detects moving gas bubbles in the main portal vein which can be visualised on B-mode images and detected by spectral Doppler as the gas bubbles reflect the sound beam overloading the system receivers giving rise to a characteristic high-pitched random bubbling sound with focal aliasing artefacts on the spectral display. The phenomenon occurs with both portal vein gas bubbles and microemboli. If sufficient gas accumulates it may become visible on CT peripherally in the portal vein branches and eventually becomes evident on plain radiographs.
Parenchymal Gas35
This is abnormal and results from a gas-forming organism in an abscess or infarct, or occasionally following trauma or hepatic arterial thrombosis following liver transplantation. It may be seen after embolisation or thermal ablation of liver tumours.
CT (Fig. 31-24) best delineates parenchymal gas collections and any related pathological changes. US will demonstrate gas collections but defining their extent may be difficult when they are large or peripheral and may be confused with adjacent bowel.
Benign Cystic Lesions
Cysts36
True hepatic cysts arise from abnormal development of bile duct precursors (Meyenburg’s complexes) and are lined by cuboidal epithelium. The true incidence is unknown and they are indistinguishable from cysts that arise as the long-term sequelae of parenchymal haematomas or abscesses. Multiple cysts occur as part of adult polycystic disease. Hepatic cysts are rarely symptomatic, although large cysts may cause pain, become infected or suffer internal haemorrhage.
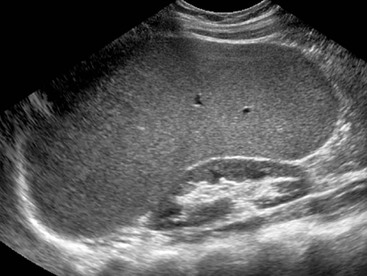
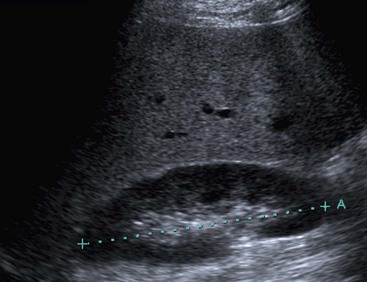
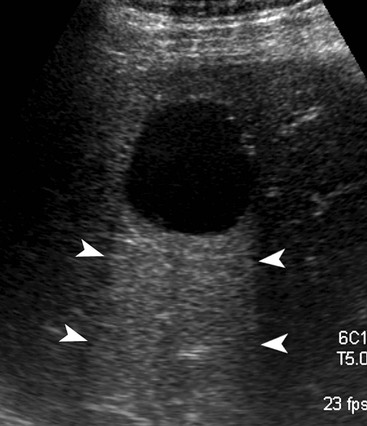
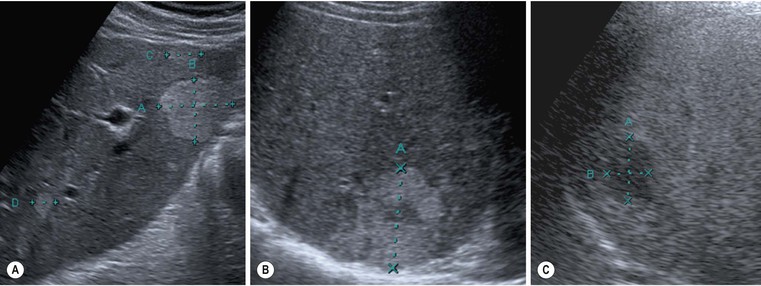
On US, hepatic cysts are spherical homogeneous structures with an imperceptible wall, posterior acoustic enhancement, lacking internal echoes and internal flow on Doppler (Fig. 31-25). A confident diagnosis may be made when these criteria are all met in a patient who does not have ovarian metastases or hydatid disease, as these conditions can mimic simple hepatic cysts. Internal echoes, thick septations, a perceptible wall or solid components should prompt further imaging (by CT or MRI) or aspiration as the differential diagnosis includes haemorrhage, abscess, cystic metastasis (e.g. ovarian), biliary cystadenoma or cystadenocarcinoma and hydatid disease. CT demonstrates cysts as homogeneous structures, with imperceptible walls, attenuation of 0–10 HU, and no enhancement following IV contrast medium. Difficulties arise with small lesions when partial volume effects may efface the characteristic features and US may be helpful to exclude a solid lesion. Occasionally cysts are of higher attenuation due to a high protein content in the fluid attributed to previous infection or haemorrhage. In these cases the lack of enhancement and features on other investigations help confirm the diagnosis. On MRI the fluid content of a cyst results in low signal on T1w imaging and very high signal on T2w imaging (particularly when using extended echo times or single shot echotrains), typically brighter than the spleen and comparable to the CSF or the gallbladder bile (Fig. 31-26). Cysts may be indistinguishable from haemangiomas on conventional T2w MRI but heavily T2w imaging (as used for MRCP) may help separate them. There is no enhancement with IV Gd-DTPA on T1w images. Scintigraphy will demonstrate large cysts as non-specific photopenic regions.
Hydatid Disease37
This is a hepatic infection with Echinococcus granulosus, a parasitic tapeworm present worldwide and transmitted from sheep, foxes and other wild animals to humans as part of its life cycle. Larvae migrate from the gut and embed in the liver, where they encyst and develop, slowly provoking a surrounding inflammatory reaction. The disease may remain occult for several years. On imaging there is a wide range of appearances, from a simple cyst indistinguishable from a true hepatic cyst to a complicated cyst with any or all of the following features: debris (hydatid ‘sand’ made up of dead scolices, which may calcify), daughter cysts, membrane separation, and wall calcification. The lesions may be multiple and vary widely in size. Serological testing confirms the presence of infection prior to any therapy or intervention. Although the risk of anaphylaxis following aspiration or surgery of these lesions is well recognised, it is less than previously thought, and uncomplicated aspiration following medical treatment has been described.
US demonstrates clearly not only the simple cyst form but also the more complex cyst features, such as the dependent debris, daughter cysts (cyst within a cyst appearance), membrane separation and wall calcification. CT defines all these features as well (Fig. 31-27) and is helpful where wall calcification obscures the view on US. MRI also defines the cystic structure and internal anatomy but is insensitive to the calcification.
Abscess38
Hepatic pyogenic abscesses usually arise from portal pyaemia. The increasing number of chronically and transiently immunocompromised patients has led to both fungal and mycobacterial abscesses becoming more common. An initial local inflammatory reaction is followed by progressive central liquefaction with a surrounding inflammatory margin or ‘wall’. In the early stages abscesses may mimic solid tumours such as metastases on virtually all imaging techniques and aspiration or biopsy may be necessary for diagnosis. The mortality from hepatic abscess has decreased with more rapid diagnosis and prompt intervention. Modern management usually involves radiologically guided diagnostic aspiration and/or drainage combined with prolonged medical therapy; surgical intervention is now rarely required.
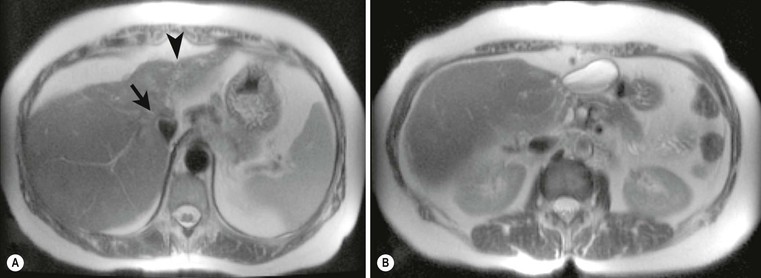
On US studies an early pyogenic abscess appears as a solid spherical lesion with an ill-defined margin and low reflectivity. As the abscess liquefies, a thickened and irregular wall appears and the necrotic centre contains sparse echoes from the debris (Fig. 31-28). On CT, abscesses are typically ill-defined, low attenuation and following IV contrast medium demonstrate rim enhancement (Fig. 31-29), although this may not occur if antibiotic treatment has started. These appearances are not specific and similar findings may be seen with metastatic deposits, particularly those with central necrosis or cystic components. The MRI findings also overlap with necrotic metastases with an ill-defined lesion on low signal on T1w and high signal on T2w, often with a higher signal outer margin. As the lesions liquefy, the central signal decreases on T1w and increases on T2w imaging.
Malignant Cystic Lesions
Metastases
Some metastatic lesions have a predominantly cystic appearance. This may occur with ovarian metastases, but has also been described with teratomas, colonic and metastatic squamous cell tumours.29 Differentiation from an abscess may be impossible on imaging criteria alone and guided aspiration for cytology and microbiology examination may be required.
Benign Solid Lesions
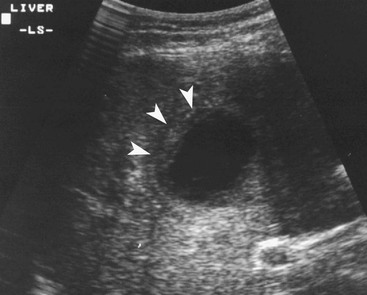
Haemangioma39–41
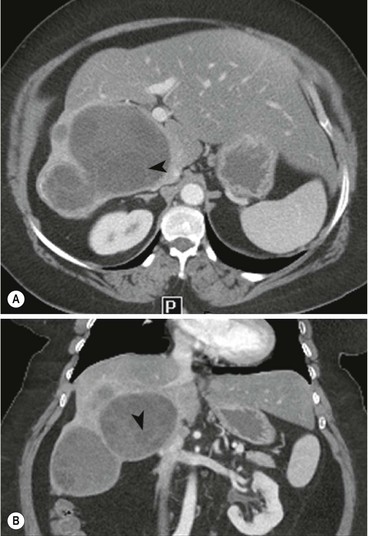
Haemangiomas are the commonest benign hepatic tumours with a postmortem prevalence of 4–20% and may be multiple in 10% of these. They are composed of vascular channels of varying size (cavernous to capillary), lined with endothelium, often with intervening fibrous tissue. Most haemangiomas are asymptomatic incidental imaging findings. Haemangiomas between 2 and 4 cm in diameter are most likely to possess characteristic features that facilitate a confident imaging-based diagnosis.
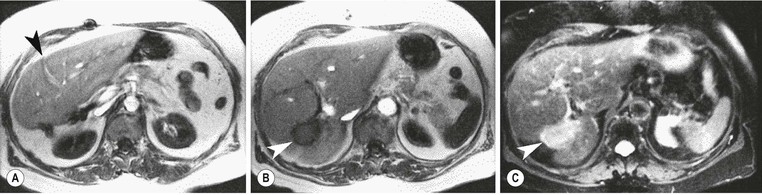
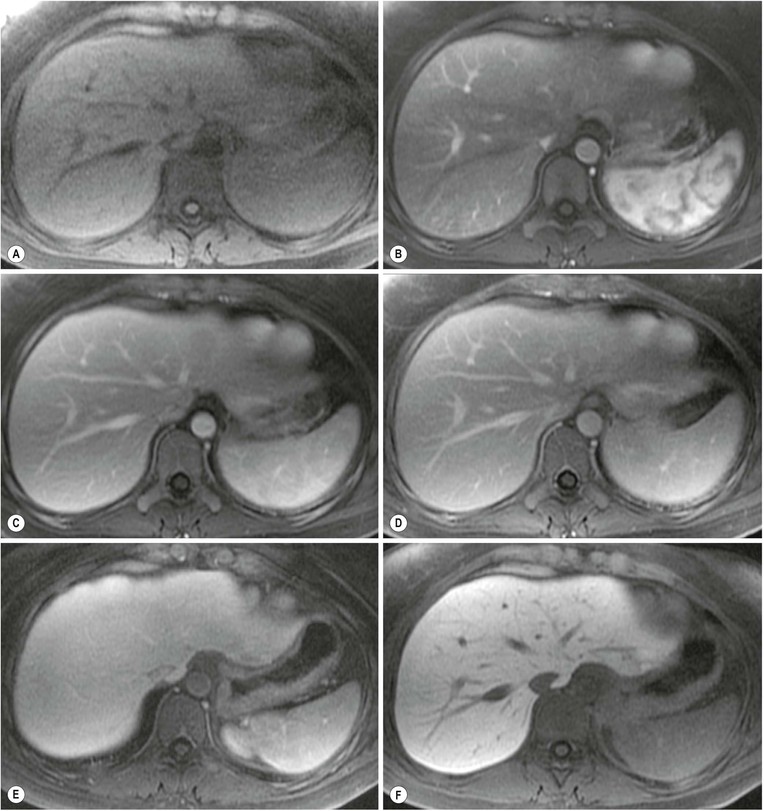
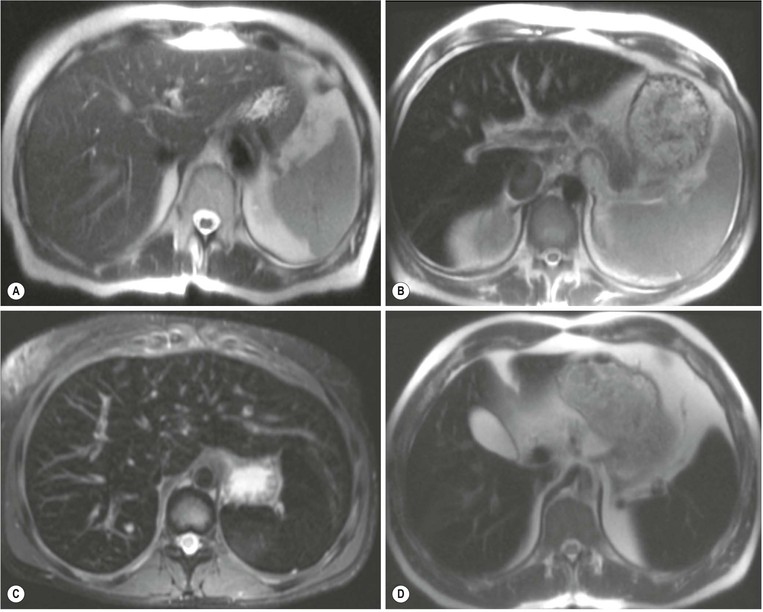
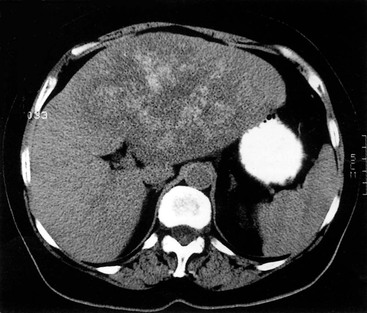
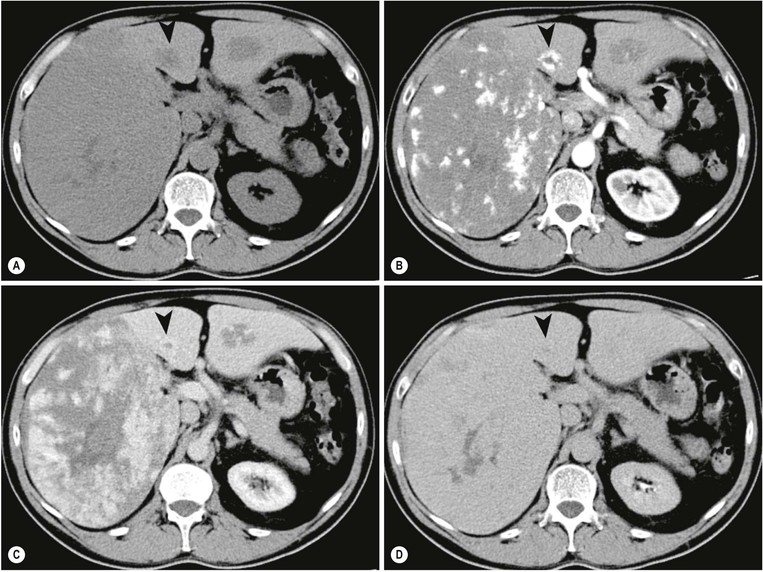
MRI is the most sensitive and specific imaging examination for the diagnosis of haemangioma. Using extended echo time (e.g. TE of 120 to 160 ms) T2w spin-echo sequences at 1.5 T, haemangiomas appear as well-defined lesions with a lobular outline and homogeneously high signal on T2w, in excess of the spleen and approaching that of fluid (Fig. 31-30). Most malignant lesions, by comparison, have signal similar to that of the spleen and become less visible on longer echo time images, unlike haemangiomas. Single-shot RARE sequences with a T2 contrast response that emphasises long T2 values may prove even more accurate for evaluation. During the arterial phase following IV enhancement with Gd-DTPA haemangiomas have rapidly enhancing vessels at the periphery. Over a period of minutes the lesion will ‘fill in’ centripetally to become isointense or slightly hyperintense with the adjacent parenchyma (Fig. 31-31). CT examination demonstrates a well-defined, lobulated lesion with attenuation close to blood values before enhancement. The pattern of enhancement follows that for MRI, with centripetally infilling and eventually merging with the background parenchyma (Fig. 31-32). Complete infilling has been applied as a diagnostic criterion, but is influenced by lesion size, with larger lesions taking 10 min or more to opacify. On US capillary haemangiomas are typically well-defined, lobular, homogeneous lesions with increased echo reflectivity (Fig. 31-33). There is usually no detectable Doppler signal within the lesion due to the slow flow, although signals may be detected in adjacent feeding vessels or within the lesion with more sensitive harmonic imaging techniques. Unfortunately some metastases, especially from neuroendocrine malignancies, may have a similar appearance. Some adult and most neonatal and infantile haemangiomas are of the cavernous type, with reduced echo reflectivity, probably due to the larger vascular channels found within them. In these lesions Doppler signals are usually detectable due to more rapid flow rates. Haemangiomas appear as photopenic regions on liver sulphur colloid studies but show an increase in uptake on blood pool studies (e.g. 99mTc-labelled red cells). This technique was widely used before the advent of MRI but is now only used where CT and MRI are unavailable.
Atypical Haemangiomas39–
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree