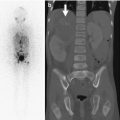
Fig. 13.1
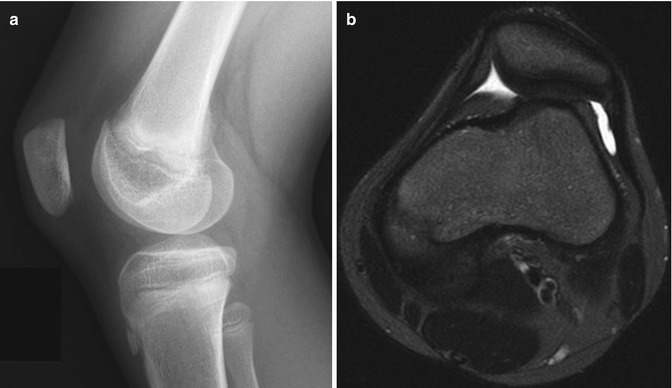
Lucencies in the central portion of the distal femoral epiphysis (white arrow) of an 8-year-old boy
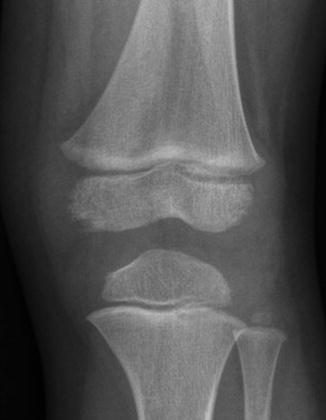
Fig. 13.2
Irregularities at the periphery of the medial (and, to a lesser extent, lateral) femoral condyle in a 3-year-old boy
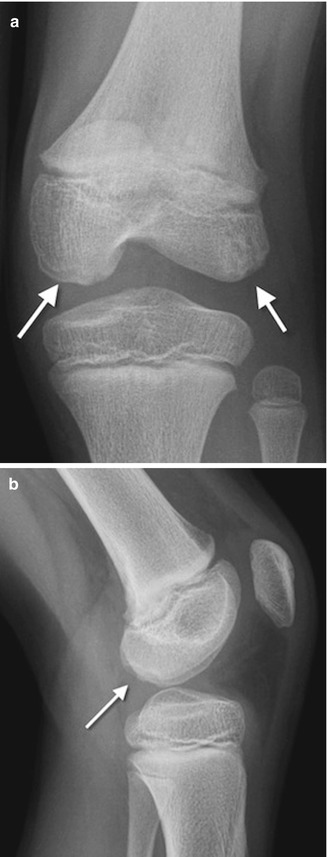
Fig. 13.3
Developmental irregular contours of the femoral condyles in an 8-year-old boy. (a) The irregularities are on the outer margin of the condyles (white arrows). (b) The defects in the contour project posteriorly and are not on the weight-bearing surface
The normal-variant depressions are often only evident on a tunnel radiograph that projects the posterior condyle in tangent and on a lateral view where they are clearly posterior. These irregularities resemble osteochondritis dissecans (OCD) but are differentiated by their position. OCD is located anteriorly or directly superior to the intercondyloid eminence inferior weight-bearing surface (Fig. 13.4), whereas the developmental variant is positioned posteriorly and does not affect the notch. These variants are present in almost one-third of young children. Note that it is occasionally impossible to distinguish variant ossification from OCD on radiographs. However, with the developmental variant, MRI demonstrates normal signal in the overlying cartilage and adjacent femoral condyle (Fig. 13.5). (See Chap. 14 for additional discussion of OCD.)
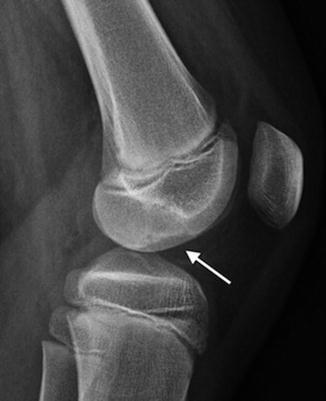
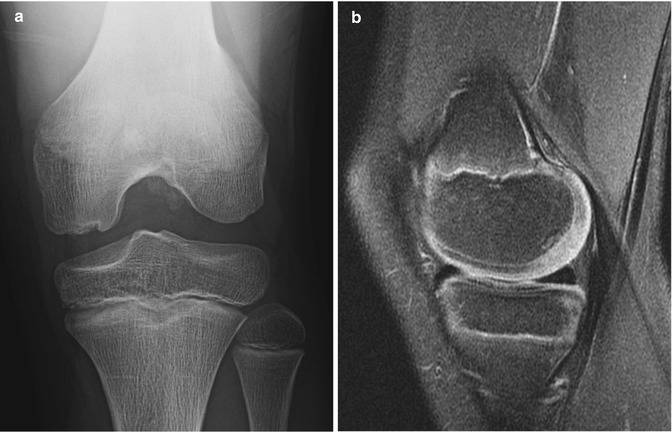

Fig. 13.4
Osteochondritis dissecans (OCD) in an 11-year-old boy. Unlike developmental irregularity of the femoral condyle, OCD is located on the weight-bearing surface (arrow)

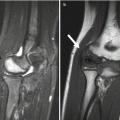
Fig. 13.5
Normal irregular ossification of medial femoral condyle in an 11-year-old. (a) Tunnel view shows irregular contour. (b) Sagittal proton density (PD) fat-suppressed (FS) image shows normal overlying cartilage and no marrow edema, differentiating this from OCD
The appearance of the articular cartilage at the knee changes with maturation (Fig. 13.6). In infancy, signal intensity is homogeneous. As the child begins walking, signal decreases in the weight-bearing portion of the cartilage. By preschool age, the posterior portion of the condyle develops areas of stippled increased signal intensity, which eventually coalesce. These may be attributed to irregular ossification, as chondrocytes hypertrophy [2]. In the mature knee, the signal intensity of non-weight-bearing cartilage is relatively T2-hyperintense, whereas weight-bearing cartilage is hypointense. (See Chap. 2 for images and additional discussion of cartilage maturation.)
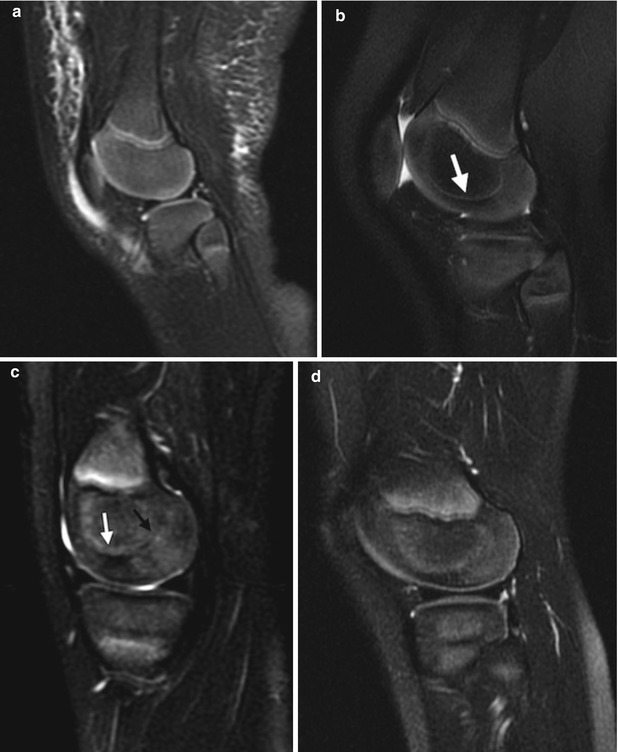

Fig. 13.6
Typical appearance of maturation of cartilage at the distal femoral epiphysis on T2-weighted (T2-W) magnetic resonance imaging (MRI). (a) At 9 months old, there is homogeneous high signal almost entirely throughout the epiphysis. (b) At 20 months old, the ossific nucleus is larger, and the weight-bearing portion is hypointense (arrow). (c) In a preschool-aged child, the posterior cartilage now appears heterogeneous and stippled, whereas the weight-bearing portion is even more hypointense (arrow). (d) With further maturation, the posterior cartilage becomes homogeneous
Nonossifying Fibroma/Fibrous Cortical Defect
Nonossifying fibromas are very common benign, eccentric, cortically based lesions with an incidence as high as 40 % in boys and 20 % in girls. Most are asymptomatic. They belong to a continuum with fibrous cortical defects and are differentiated by size (fibrous cortical defects are smaller, less than 2 cm). These cortical lucencies are found close to the physis in younger children but appear to move toward the diaphysis with progressive bone growth (Fig. 13.7). Epiphyseal involvement has not been reported. They are not usually encountered before 18 months, peak at 5 or 6 years of age, and are rarely evident in adults. Lesions are commonly on the dorsal and medial aspect of the bone and may be bilateral. The vast majority occur in the lower extremity, and 82 % occur around the knee. They may be found simultaneously in the femur, tibia, and fibula.
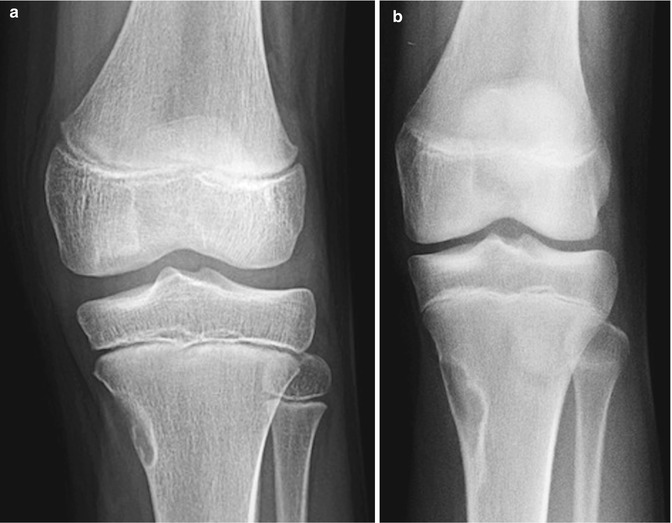

Fig. 13.7
Nonossifying fibroma. (a) Cortically based lesion with sclerotic margin and narrow zone of transition, without osseous matrix. (b) Four years later, the lesion is larger but retains benign characteristics. It is positioned further from the physis
These benign lesions probably originate at sites of tendinous or ligamentous insertion into the physeal perichondrium [3]. The traction force can be estimated by drawing a line toward the joint, along the long axis of the lucency. A defect in the dorsal medial femur thus results from traction by the adductor magnus muscle or medial gastrocnemius head; a defect in the dorsal medial tibia is due to pull from the semimembranosus muscle.
Imaging
When viewed en face, the defects appear as round, oval, multicystic, or irregularly marginated lucencies, often with sclerotic edges. When viewed tangentially on a lateral projection, they are almost invariably located in the posterior cortex, projecting either completely within the cortex or as shallow defect in its outer layer, and their greatest longitudinal axis parallels the long axis of the bone. They have a narrow zone of transition and lack aggressive features.
With longitudinal growth, the lesions appear to migrate away from the joint. Although initially ovoid and only faintly demarcated, they often become polycystic, with well-defined sclerotic margins and slight expansion of their thin cortical rim. During maturation the central lucency fills with sclerotic bone, beginning at the diaphyseal end. Once sclerosis is complete, the defect remodels to normal bone and usually disappears.
Radiographs are usually sufficient for diagnosis. Additional imaging should be performed only if the lesion is painful (in the absence of fracture). MRI shows lesions are initially low to intermediate on T1-weighted (T1-W) sequences and may initially demonstrate increased signal on T2-weighted (T2-W) images. As they involute, they become hypointense on all sequences (Fig. 13.8) [4]. The central tissue shows mild enhancement after gadolinium administration [3].
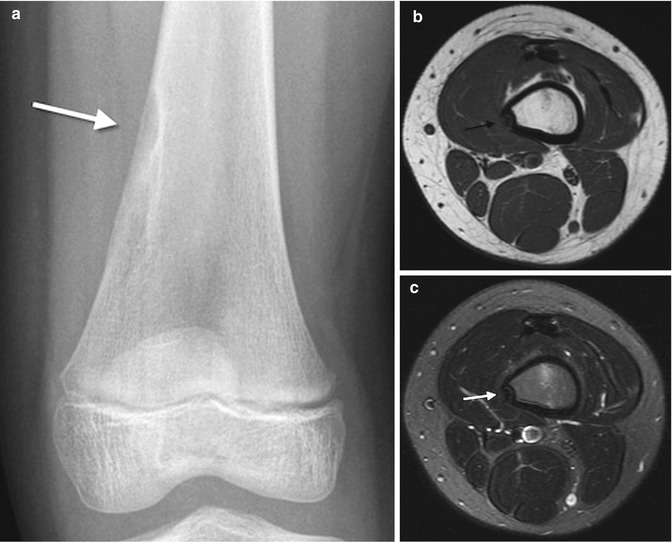

Fig. 13.8
Involuting nonossifying fibroma. (a) There is lucent scalloping within cortical sclerosis (arrow). Corresponding axial T1-weighted (T1-W) (b) and T2-W (c) images show focal subcortical hypointensity (arrows)
Stress fractures have been reported in association with nonossifying fibromas and fibrous cortical defects [5] (Figs. 13.9 and 13.10). Fractures are more likely with larger lesions that occupy more than half of the bone width, extending into the marrow cavity (Fig. 13.11).
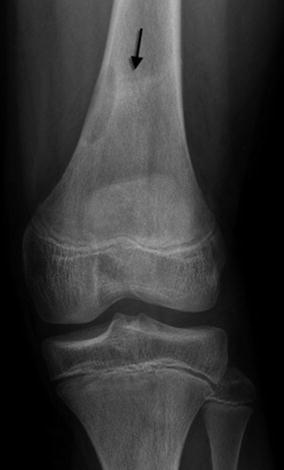
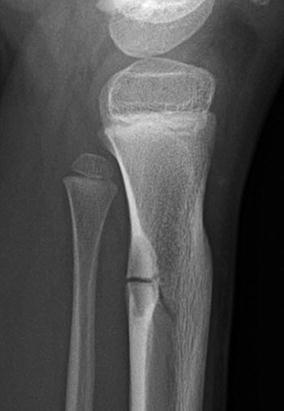
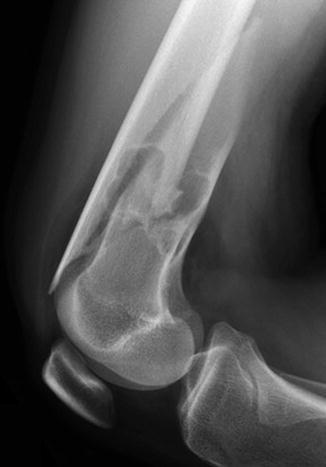

Fig. 13.9
Healing stress fracture through nonossifying fibroma. Linear sclerosis (arrow) extends laterally from the middle of the lesion

Fig. 13.10
Stress fracture through nonossifying fibroma in a 6-year-old boy. Stellate linear lucency traverses a cortically based lesion

Fig. 13.11
Pathological fracture through a large nonossifying fibroma
Differential diagnoses include fibrous dysplasia, eosinophilic granuloma, and simple bone cysts. However, radiographic evaluation should show that benign cortical lesions are limited to the cortex, excluding these other diagnoses. In some cases, it may be difficult to completely exclude potentially intracortical lesions such as Brodie abscess or periosteal chondroma. Multiple nonossifying fibromas/fibrous cortical defects occur in neurofibromatosis and Jaffe-Campanacci syndrome [6]. (See Chap. 21 for further discussion.)
Posterior Metaphyseal Stripe
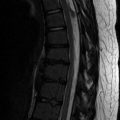
A hyperintense band of tissue is common along the subperiostium at the posterior aspect of the distal femoral metaphysis as well as along the posterior aspect of the proximal tibial metaphysis. This band, composed of loose fibrovascular connective tissue, is more prominent on the femur than on the tibia. It is seen only in skeletally immature patients and on proton density (PD), T2-W, and inversion recovery (IR) sequences (Fig. 13.12); it is not evident on T1-W images. The tissue probably contributes to dynamic bone modeling that accompanies skeletal growth [7].
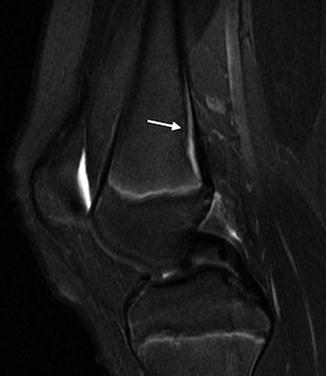

Fig. 13.12
Posterior metaphyseal stripe. Sagittal T2 FS sequence demonstrates linear subperiosteal hyperintensity along the posterior distal femoral metaphysis
Cortical Desmoid
Cortical desmoids occur at the posteromedial metaphysis of the distal femur, at the insertion site of the medial head of the gastrocnemius muscle. This cortical roughening is common between ages 10 and 15, occurring in about 11 % of boys and 3 % of girls [8]. The lesion is benign, self-limited, and usually asymptomatic, although there may be associated pain [9].
Etiology is uncertain. The lesion may represent a remodeling response to chronic or acute traction injury. It has also been theorized to result from chronic tension at the attachment of the medial head of the gastrocnemius or the adductor magnus aponeurosis, leading to the term “tug lesion.” Very rapid growth may contribute as well. Subperiosteal connective tissue and osteoclasts within a cortical erosion have been identified at the attachment of the adductor magnus muscle [10]. Lesion shape may differ depending on the orientation of mechanical traction [9].
Imaging
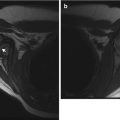
Cortical desmoids are located in the posteromedial cortex, 1–2 cm proximal to the growth plate, and thus just above the adductor tubercle. Radiographic findings range from faint cortical fuzziness (Fig. 13.13) to irregular margination to osseous spiculation perpendicular to the cortex, characteristic of rapid periosteal bone formation (Fig. 13.14). A scalloped cortical defect or bony prominence is often apparent (Fig. 13.15). Desmoids are most evident on a slightly externally rotated projection so that the posteromedial supracondylar area is projected in tangent (see Fig. 13.13).
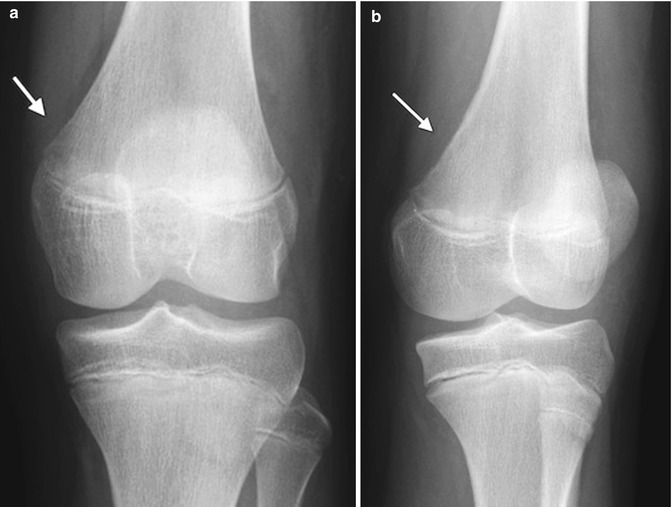
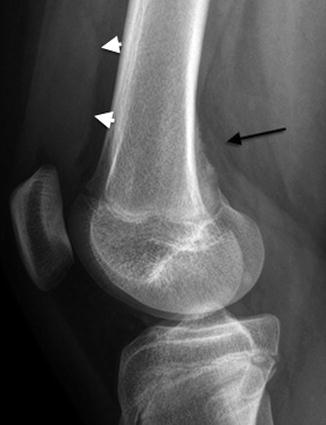
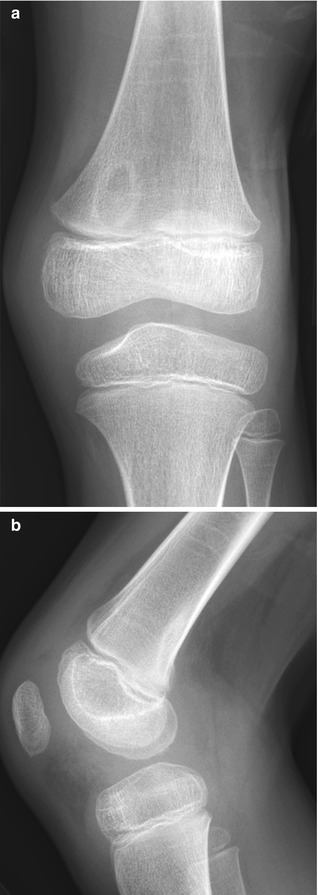

Fig. 13.13
Cortical desmoid. (a) Ill-defined lucency on frontal view. (b) Subtle cortical irregularity on projection obtained with slight external rotation

Fig. 13.14
Cortical desmoid in a 12-year-old girl. There is slight cortical irregularity (arrow) on the medial aspect of the posterior femur, at the site of the origin of the medial head of the gastrocnemius. Note unrelated joint effusion (arrowheads)

Fig. 13.15
Cortical desmoid in a 7-year-old boy. (a) Frontal projection demonstrates ovoid lucency with sclerotic margins. (b) Lateral shows posterior sclerosis with scalloped lucent defect (Courtesy of Tal Laor, Cincinnati Children’s Hospital)
At MRI, these lesions appear as T1-hypointense and T2-hyperintense cortical scalloping, with a dark rim of bony sclerosis along the attachment of the medial head of the gastrocnemius muscle (Fig. 13.16) [9]. If there has been acute trauma, marrow and intratendinous edema may be evident.
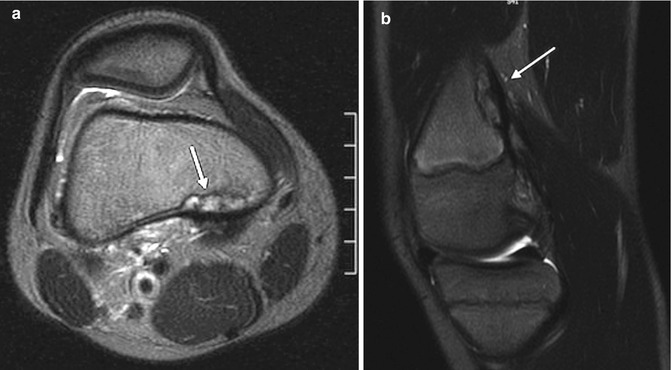

Fig. 13.16
Cortical desmoid. (a) Axial T2-W image shows heterogeneous signal along posteromedial distal femoral metaphysis, demarcated by hypointense cortex (arrow). (b) Sagittal T2-W image shows insertion of the medial head of the gastrocnemius muscle (arrow)
Although the appearance may resemble periosteal reaction with bony erosion, cortical desmoids should not be mistaken for a malignant or inflammatory process. If a pathological process is suspected, nuclear scintigraphy will not necessarily help differentiate, especially if the appearance is caused by a minor avulsion that might also generate increased radiotracer uptake. MRI may help confirm the benign nature of this lesion. Cortical desmoids are differentiated from nonossifying fibromas/fibrous cortical defects because the latter appear to migrate as the patient grows and also erode the cortex from within the bone, whereas cortical desmoids do not change position and do disrupt the outer surface [4].
Tibial Tuberosity
The tibial tuberosity forms from an anteroinferior cartilaginous extension of the tibial epiphysis. Before ossification of the tuberosity begins, it can appear as a large, abruptly scalloped defect, which can be smooth or show mild undulation (Fig. 13.17). It may mimic an erosive lesion or a buckle fracture (Fig. 13.18) [11]. Ossification begins as a distal extension of the primary epiphysis. However, a separate center occasionally develops and grows concentrically, eventually uniting with the more proximal portion of the epiphysis. The tuberosity fuses with the adjacent bone by age 15 in girls and age 19 in boys.
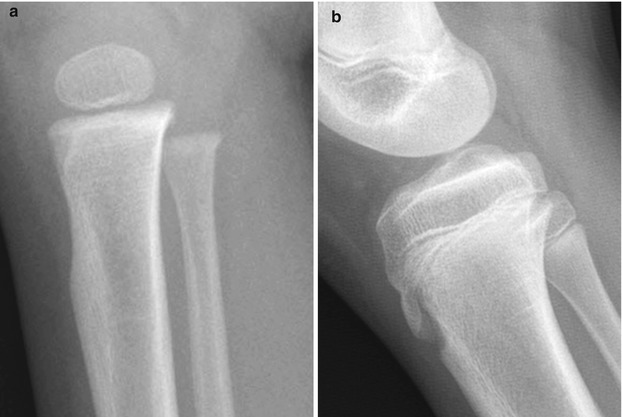
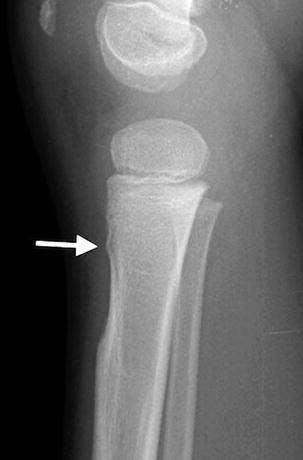

Fig. 13.17
Development of the tibial tuberosity. (a) In early childhood, the purely cartilaginous tibial tuberosity results in concavity of the anterior surface of the proximal tibia. (b) As the tuberosity ossifies, continuity with the proximal tibial metaphysis becomes apparent

Fig. 13.18
Buckle fracture of the proximal tibia in a 3-year-old boy (arrow)
In most cases, the developing center is either multicentric or irregular. Lack of soft tissue swelling and clinical complaints help differentiate it from an avulsion fracture or Osgood-Schlatter disease (Fig. 13.19).
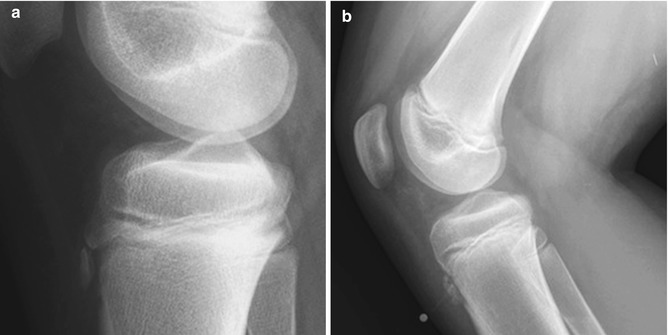

Fig. 13.19
Fragmentation of the tibial tuberosity. (a) Normal fragmentation of the tuberosity, with no overlying soft tissue swelling. (b) Osgood-Schlatter disease, with soft tissue swelling and thickened patellar tendon (metal marker indicates focal tenderness)
Growth Recovery Lines
Growth recovery lines appear as discrete horizontal lines of sclerosis parallel to the growth plate, developing after temporary growth cessation. They are quite common in both femoral and tibial metaphyses and are often present simultaneously at multiple metaphyses that significantly contribute to bone growth. They usually extend in a continuous line across the metaphysis and gradually remodel, apparently moving toward the diaphysis with continued growth (Fig. 13.20). While it seems logical that growth arrest lines would occur more often in children with frequent severe illnesses, in fact they are also common in healthy children or in those who have suffered the usual minor childhood illnesses. Persistence into early adulthood indicates a previous vigorous recovery response rather than chronic illness. Focal deformity of a growth arrest line can provide important information about adjacent growth arrest following physeal injury, before development of bony bridging or angular deformity.
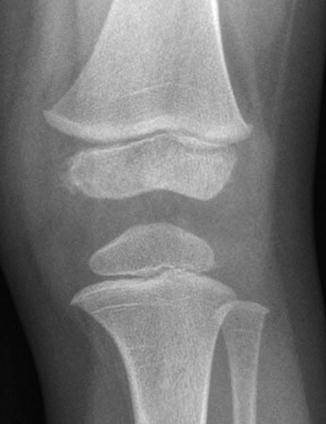

Fig. 13.20
Growth recovery lines appear as thin, sclerotic, horizontal lines traversing the metaphyses of all the bones about the knee
The Fabella
The fabella is an ovoid, occasionally multipartite, sesamoid bone in the lateral gastrocnemius muscle belly (the tendinous portion of this muscle is distal) (Figs. 13.21 and 13.22). It is found in about 12 % of the population and is bilateral in more than half [12]. Although the fabella is almost always of no clinical significance, teens occasionally complain of pain with full extension as well as local tenderness, and this may be relieved by excision of the fabella [13].
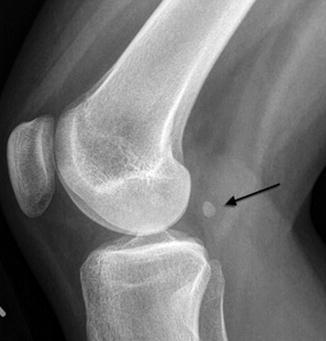
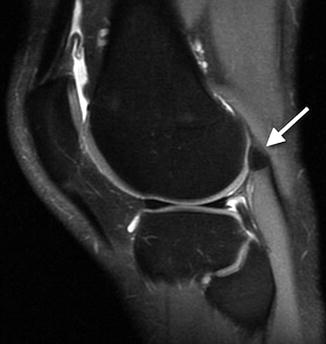

Fig. 13.21
Fabella. Black arrow indicates this sesamoid bone

Fig. 13.22
Large fabella (white arrow) within the tendon of the lateral head of the gastrocnemius (sagittal T2-W fat-suppressed (FS) image)
The Patella and Patellofemoral Articulation
The anterior surface of the bony patella has many vascular foramina and vertical striations. These appear on radiographs as lucencies or thin anterior fissures (Fig. 13.23) and on MRI as channels that parallel the long axis of the patella, generally with high signal on T2-W images (Fig. 13.24). The posterior surface of the patella has two facets, separated by a median ridge (Fig. 13.25). At birth the lateral facet is already larger than the medial, and it ultimately becomes 40 % longer. The lateral facet is less steeply angled. The angle between the two facets averages 130°. The upper two-thirds of the posterior surface is covered with articular cartilage (Fig. 13.26), and—as the thickest cartilage in the mature body—it may be up to 5 mm deep in the adult.
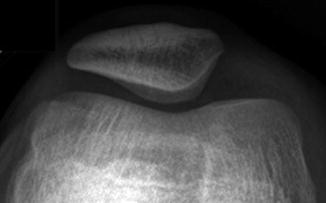
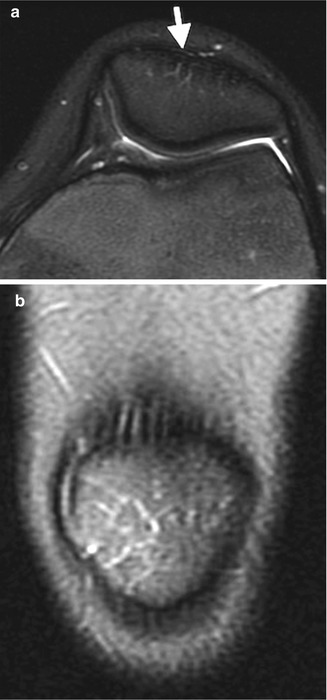
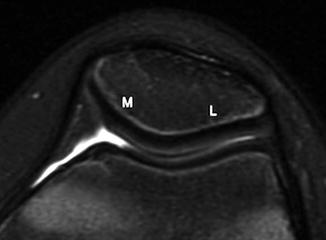
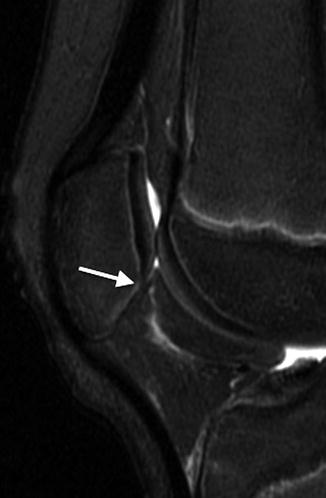

Fig. 13.23
Vascular grooves at the anterior patella. There are lucent striations at the anterior surface of the patella

Fig. 13.24
Vascular grooves at the anterior patella. Axial (a) and coronal (b) T2-W images show hyperintense branching vessels (arrow)

Fig. 13.25
Normal patella with a prominent ridge dividing the smaller medial (M) facet from the larger lateral (L) facet (axial T2-W image)

Fig. 13.26
Normal patella with articular cartilage covering only the upper two-thirds of the posterior surface (arrow denotes transition between cartilage and bone) (sagittal T2-W image)
The shape of the patella, the shape of the femoral condyles posterior to the patella, and the configuration of the proximal tibia affect the likelihood of developing patellar derangement. The patella lies in a sulcus that is a superior extension of the intercondylar notch. The sulcus is formed by the anterior contours of adjacent portions of the medial and lateral femoral condyles. The lateral surface of the sulcus projects more anteriorly than does the medial, restricting lateral patellar subluxation. (The force vectors of the quadriceps muscles apply both a major component of longitudinal force on the patella and a smaller component of lateral force: this lateral force may contribute to patellar subluxation.) Thus, the lateral facet of the femoral trochlea plays an important role in helping to stabilize the patellofemoral articulation. A shallow sulcus, as seen in children with longitudinal deficiency of the fibula, can contribute to instability.
Another important factor in evaluating patellar stability is alignment of the patella with respect to the tibial tuberosity. This can be assessed in several ways. The Q angle is calculated on MRI from the midline of the quadriceps as it inserts into the patella to the patellar tendon as it aligns with the tibial tuberosity. Alternatively, the tibial tuberosity to trochlear groove (TT-TG) distance measures the space between the midpoint of the tibial tuberosity and the deepest part of the trochlear groove (described in detail in section below on developmental patellar instability and dislocation).
The suprapatellar pouch is usually an extension of the knee joint and very rarely a bursa that does not communicate with the joint. There is a small amount of fat anterior to this pouch, posterior and superior to the patella, and a larger amount of fat posterior to it, anterior to the femur. On a lateral knee radiograph, the pouch appears as soft tissue density outlined by fat. A joint effusion is present if the soft tissue between these two fat pads exceeds 10 mm in thickness (Fig. 13.27). If this distance is between 5 and 10 mm and the margins of the recess are fuzzy, an effusion is suggested.
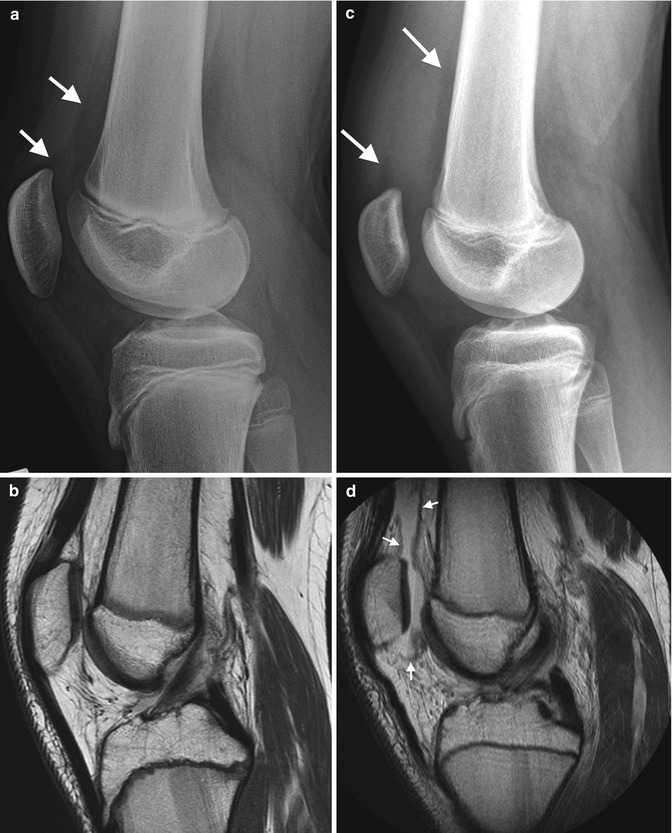

Fig. 13.27
Joint effusion. Cross-table lateral view (a) in a normal 12-year-old boy (for comparison) shows a normal, thin suprapatellar pouch, outlined by fatty tissue (arrows). (b) Accompanying sagittal PD MRI. (c) Hemarthrosis in a 14-year-old boy with tibial spine fracture and anterior cruciate ligament (ACL) tear shows the fat pads (arrows), along with thickening of the suprapatellar pouch. (d) PD MRI shows hemarthrosis (short arrows) with fluid-fluid level
The patella serves many functions: it decreases friction from the quadriceps around the femoral trochlea, transmits the vectors of the quadriceps system into one patellar tendon, protects against dislocation of the tendon from within the sulcus, and shields knee cartilage from injury.
Ossification variants are common. The ossified patella may be granular or nodular; it may have zones of sclerosis and lucency. Anterior lucencies as well as transverse and vertical anterior surface ridges are particularly common. Vertical fissures (larger than the thin branching fissures discussed above (see Figs. 13.23 and 13.24)) are most common (Fig. 13.28). Most variants are isolated, but they may mimic findings in multiple epiphyseal dysplasia or be confused with a patellar sleeve fracture.
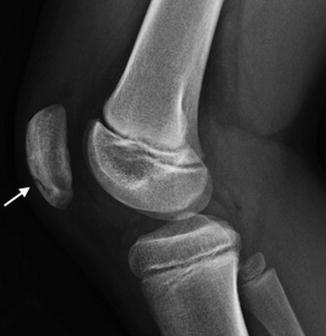

Fig. 13.28
Vertical patellar fissure (white arrow)
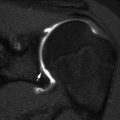
A bipartite patella is the most common variation in patellar ossification, estimated to occur in 1–6 % of the population, and is slightly more likely to be unilateral than bilateral. The patella may form from multiple ossification centers, and if they fail to fuse a bi- (or tri-) partite patella results. The extra center is usually superolateral (Fig. 13.29), but in 20 % it is lateral and in 5 % at the lower pole (Fig. 13.30). There may be pain and increased mobility at the cartilaginous interspace adjacent to an accessory center.
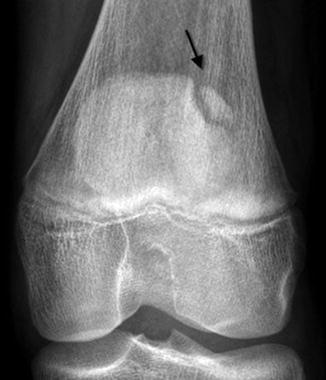
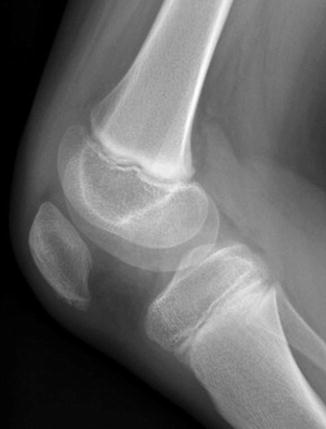

Fig. 13.29
Bipartite patella, most common at the superolateral patella (arrow)

Fig. 13.30
Accessory ossification center at the lower pole of the patella in a 6-year-old boy
It is possible that some bipartite patellae result from stress fracture rather than developmental variation [14]. In addition, several pathological entities mimic the inferior accessory center at the lower pole. It may be confused with a traumatic patellar sleeve fracture (Figs. 13.31 and 13.32) a hyperflexion injury involving both cartilage and bone. If this fracture affects an incompletely ossified patella, it may be subtle, involving only the cartilaginous portion of the lower pole. In the setting of fracture, the patella is often high riding, with a small fragment avulsed from the inferior pole. Radiography underestimates the extent of cartilaginous involvement, and MRI is often necessary [15].
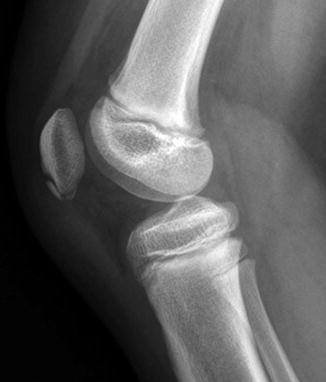
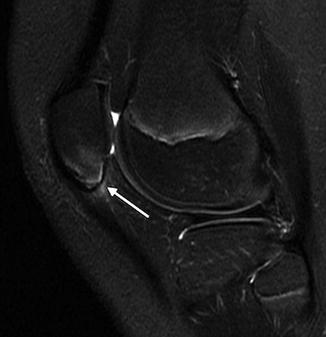

Fig. 13.31
Patellar sleeve avulsion fracture with two large fragments avulsed from the osseous lower pole. Note the associated soft tissue swelling in Hoffa’s fat as well as thickening of the patellar tendon

Fig. 13.32
Patellar sleeve avulsion fracture in a 12-year-old boy. Sagittal T2-W image shows transverse fracture through the lower pole of the patella with associated soft tissue edema. Arrow indicate avulsed fragment
Another differential consideration is Sinding-Larsen-Johansson disease. This is most common in children between the ages of 10 and 14 and presents with restricted knee motion as well as localized tenderness and soft tissue swelling over the lower patella. As with Osgood-Schlatter avulsion, this is neither a “disease,” osteochondritis, inflammation, nor avascular necrosis but is almost certainly traumatic. Radiographic findings of Sinding-Larsen-Johansson include an accessory ossification center or avulsed fragments at the lower border of the patella, with associated soft tissue swelling (Fig. 13.33). The appearance on both radiographs and MRI is essentially indistinguishable from patellar sleeve fracture, and differentiation relies on clinical history and examination.
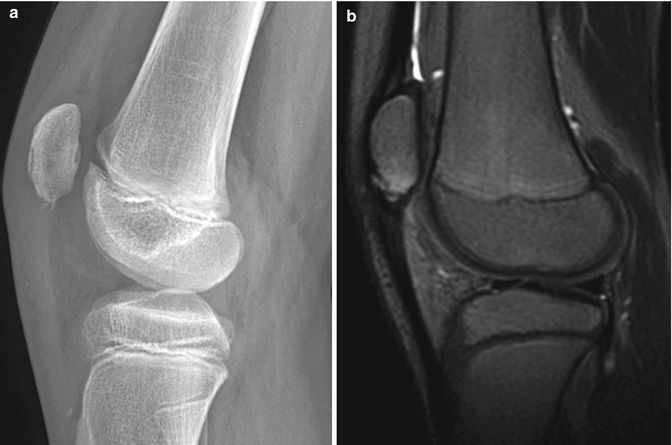

Fig. 13.33
Sinding-Larsen-Johansson disease in an 11-year-old boy with anterior knee pain. (a) The lower pole of the patella is slightly fragmented. (b) Sagittal T2-W FS image confirms fragmentation and shows mild bone marrow edema and patellar tendon thickening
Cerebral palsy is also associated with avulsion fractures of the lower pole of the patella, resembling an inferior patellar ossification center. There may be one or several fragments, separated from or adjacent to the patella. In spastic ambulatory children with cerebral palsy and flexion contractures, the patella may be high, elongated, and crescent shaped (see Chap. 24). The appearance probably reflects recurrent mechanical overload of the knee extensor mechanism with healed, subclinical avulsion fractures or ligamentous micro-tears. Ultrasound (US) can demonstrate patellar tendon thickening and local edema, probably due to contusion over (or avulsion of) an accessory center, a fracture-avulsion of the lower patella, or simply contusion of the patellar ligament with ligamentous ossification.
2 Patellar Abnormalities
2.1 Patellar Agenesis
A completely absent patella is rare as an isolated finding, generally running in families. Usually an absent patella indicates the patient has nail-patella (onycho-osteodysplasia, or Fong) syndrome, which consists of dysplasia of the fingernails, radial head and capitellar deformity with potential radial head dislocation, iliac horns, and renal disease. The patella has been found to be absent in approximately 15 % of patients with this disorder and to be small, angular, sclerotic, or otherwise dysplastic in about 85 % [16]. Femoral condylar dysplasia is often associated.
Patellar hypoplasia may also be encountered as an isolated variant, in families, or accompanying congenital dislocation of the patella or contracture of the quadriceps muscle. There are also many variations in facet size and orientation as well as median ridge development. Patellar and femoral cartilaginous and bony surfaces are not necessarily congruent, and radiographs may not portray the true anatomy. MRI may provide a more accurate assessment of contours. Patellar duplication is extremely rare.
2.2 Congenital Patellar Dislocation
Congenital patellar dislocation refers to lateral dislocation present at birth or shortly thereafter. It may be associated with arthrogryposis, Down syndrome, nail-patella syndrome, and other genetic syndromes. The association with Down syndrome suggests this disorder results from ligamentous laxity, but it may also be due to failed rotation of the quadriceps muscle myotome. Associated findings include anomalous insertions of fibrous bands into the lateral patella as well as tight lateral capsular structures and synovial fibrosis. Quadriceps fibrosis has also been found in many cases [17].
Infants with patellar dislocation may present with a fixed flexion contracture. However, the knee may have a normal range of motion, and the patella may dislocate only with flexion. Diagnosis is often made only after the patient begins walking. Surgery—generally release of the patella laterally and lengthening of the quadriceps tendon—should be performed promptly. Failure to recognize and treat this condition early may result in progressive flexion contracture at the knee, as well as genu valgum and torsional deformities.
Imaging
Patellar ossification does not begin until 1.5 years of age at the earliest, but its position may be deduced on radiographs by an ovoid increased soft tissue density. US can readily assess patellar position before ossification begins (Fig. 13.34). In extension, it may be normally positioned or dislocated (usually laterally). The dislocated patella may rotate 90° such that its articular surface abuts the lateral aspect of the relatively small lateral femoral condyle (Fig. 13.35). The axial projection shows a shallow sulcus, sometimes associated with hypoplasia of the femoral condyles [18]. MRI is also useful for assessing the position and configuration of a dislocated patella before it has ossified.
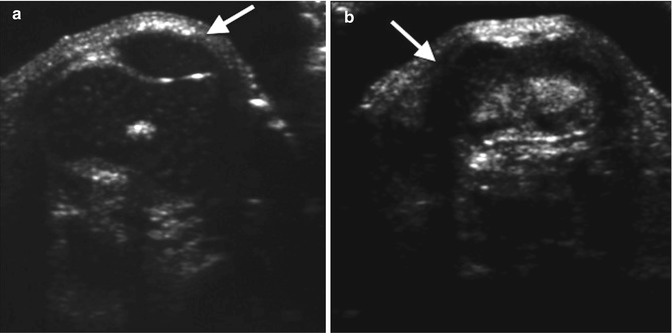
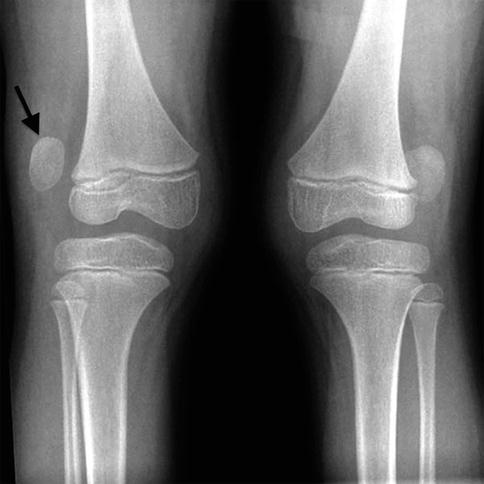

Fig. 13.34
Dislocated patella in an infant. (a) Normal side (for comparison) shows the hypoechoic patella (arrow) nestled in the intercondylar notch of the femur. (b) On the right, the distal femur is identified (arrow), but the intercondylar notch appears shallow, and the patella is not in its expected position (Courtesy of Rebecca Stein-Wexler)

Fig. 13.35
Congenital patellar dislocation in a 6-year-old with bilateral lateral dislocation of the patella. The lateral femoral condyles are relatively small, and the right patella is rotated (arrow) (Courtesy of Hank Baskin)
2.3 Developmental Patellar Instability and Dislocation
Patellar dislocation is common in young, active individuals, usually resulting from a twisting motion with the knee flexed. The annual incidence is approximately 43 per 100,000 children under age 16 years old [19]. The medial retinaculum, medial patellofemoral ligament, shape of the femoral trochlea, position of the tibial tuberosity, and the extensor muscles of the knee stabilize the patellofemoral articulation. Abnormal development of the femoral trochlea may lead to a shallow femoral trochlear sulcus, reducing the normal osseous barrier to lateral subluxation. This trochlear dysplasia thus leads to instability and recurrent patellar dislocations, and indeed 85 % of patients with patellar dislocation have imaging signs of trochlear dysplasia. In addition, an abnormally positioned tibial tuberosity may also contribute to patellofemoral instability by changing force vectors. If the medial stabilizing ligaments tear during an episode of dislocation, chronic instability results [20].
Surgery is frequently the definitive treatment. The type of surgery performed depends on imaging findings. Frequently, the stabilizing ligaments are repaired, specifically with medial patellofemoral ligament repair and medial capsular plication. A lateral capsular release is often performed as well. If lateralization of the tibial tuberosity contributes to instability, tibial tuberosity transfer can be performed after skeletal maturity (the tendon and an associated bone fragment are moved medially and reattached to the tibia). This decreases the lateral force vector of the quadriceps muscles, decreasing instability. In severe dysplasia, trochleoplasty may be performed, which involves deepening the trochlear sulcus by a curved osteotomy at the upper portion of the articular surface.
High-lying patella (patella alta) is associated with recurrent patellar subluxation, chondromalacia of the patella, Sinding-Larsen-Johansson lesion, recurrent effusion, and cerebral palsy. A low patella (patella baja or infera) occurs in achondroplasia, Osgood-Schlatter lesion, and poliomyelitis.
Imaging
Patellar position may be measured in several ways on a lateral radiograph or lateral MRI (Table 13.1). In a skeletally mature knee, the Insall-Salvati ratio and lateral trochlear inclination best predict both lateral patellar displacement and lateral tilt of the patella [21].
Table 13.1
Signs of trochlear dysplasia and patellar instability
Normal value | |
|---|---|
Lateral trochlear inclination | >11° |
Lateralization of tibial tuberosity | <15 mm |
Patellar height ratio (Insall/Salvati) | <1.4 |
Patello-tibial/femorotibial ratio (PT/FT) (Koshino and Sugimoto) | >0.9, <1.3 |
Trochlear depth | >3 mm |
Trochlear facet ratio | >0.4 |
The Koshino and Sugimoto method is best used for children under 13 years of age, whose patella may not yet have ossified completely [22]. Lines are drawn along the distal femoral and proximal tibial growth plates. A line that connects them at their midpoints forms the femorotibial (FT) distance. The patello-tibial (PT) line is then drawn from the tibial midpoint to the center of the patellar axis (PT) (Fig. 13.36). A normal patello-tibial/femorotibial (PT/FT) ratio is 0.9–1.3.
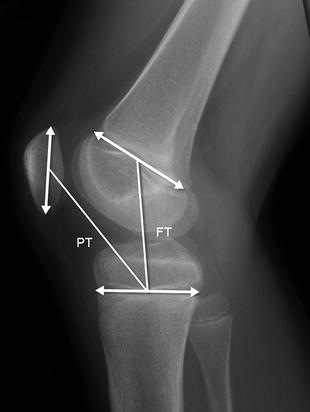

Fig. 13.36
Koshino-Sugimoto method for assessing patellar height ratio in a skeletally immature patient. The distance from the midpoint of the patella to the midpoint of the tibial physis (PT) is compared with the distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis (FT)
The Insall and Salvati method should be used on radiographs only in patients over the age of 13, as a partially cartilaginous patella will yield misleading results [23]. The length of the patellar tendon is measured along its posterior edge—from the apex of the patella to its insertion into the tibial tuberosity (Figs. 13.37 and 13.38). The diagonal length of the patella is also measured. The patellar tendon and patellar lengths should be nearly equal. Patella alta is defined as a tendon/patella ratio greater than 1.3, and patella baja is defined as a ratio less than 0.7. This ratio is accurate on MRI’s of younger children, while patellar ossification is still incomplete.
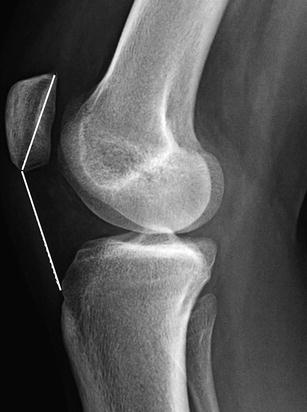
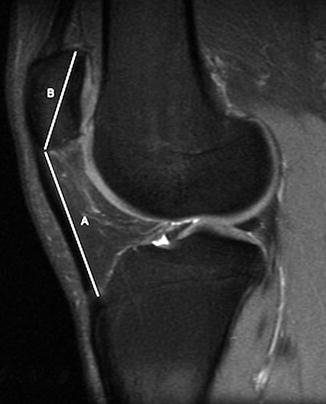

Fig. 13.37
Insall and Salvati method for assessing patellar height ratio in a skeletally mature 17-year-old girl: the diagonal length of the patellar tendon (measured from the apex of the patella to its insertion into the tibial tuberosity) is compared with the maximum diagonal length of the patella (Courtesy of Rebecca Stein-Wexler)

Fig. 13.38
Patella alta. Sagittal T2-W FS MRI shows the length of the patellar tendon (A) compared with the greatest height of the patella (B) is 1.55
Patellar position may also be assessed with respect to the Blumensaat line, which is drawn along the roof of the intercondylar notch with the knee flexed to 30° (Fig. 13.39). The position of the patella is determined by measuring the distance from the lower pole of the patella to this line. Patella alta is present if the inferior pole of the patella is more than 29 mm above this line [24]. This technique is simple to perform but less accurate than either the Koshino and Sugimoto method or the Insall-Salvati ratio.
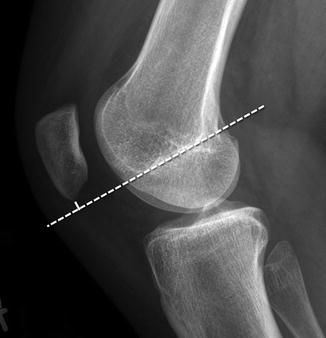

Fig. 13.39
Blumenstat method for assessing patellar position. Performed on a lateral radiograph with the knee in 30° of flexion, this technique determines the distance between the inferior tip of the patella and Blumenstat line (long dashed line). Blumenstat’s line is tangent to the intercondylar roof
The axial projection helps evaluate patellar position and angulation, as well as the depth and configuration of the sulcus. MRI best evaluates articular cartilage, ligamentous structures, and the patellofemoral articulation [20]. Trochlear dysplasia can be evaluated on axial images.
Measurements of lateral trochlear inclination, sulcal depth, and facet ratio may be useful. The lateral trochlear inclination is measured on the first axial image that includes the trochlear cartilage by determining the angle formed by two lines. One line is tangent to the lateral trochlear facet, and the second is tangent to the posterior surface of the femoral condyles (Fig. 13.40). An abnormal angle is less than 11° [25].
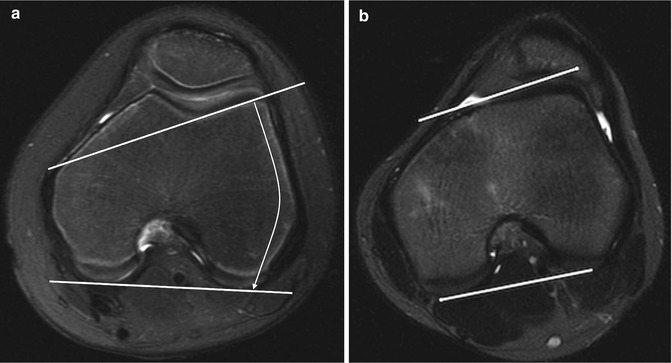

Fig. 13.40
Lateral trochlear inclination. The angle between a line tangent to the posterior aspect of the femoral condyles and a line tangent to the subchondral bone on the lateral trochlear facet. (a) Normal. (b) 10-year-old girl with abnormal trochlear inclination of 8° (axial T2-W FS MRI)
Trochlear depth is the maximum distance between a line connecting the highest points of the lateral and medial facets and the deepest point of the trochlear sulcus. This measurement is obtained 3 cm above the tibiofemoral joint cleft (Fig. 13.41). A sulcal depth less than 3 mm is abnormal [26].
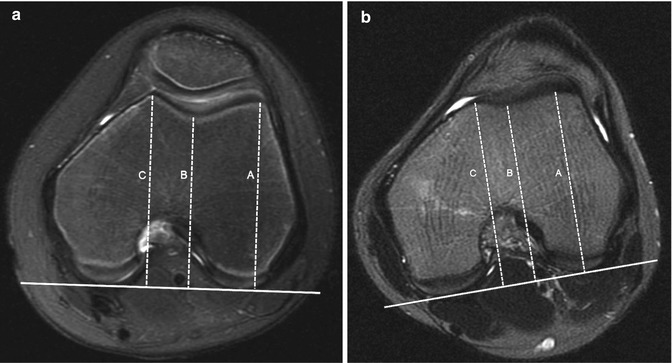

Fig. 13.41
Trochlear depth. An average of the difference between the highest point of the lateral (A) and medial (B) facets and the deepest point of the trochlear sulcus (C). (a) Normal. (b) Ten-year-old girl with very shallow trochlea, 1 mm deep (axial T2-W FS MRI)
The trochlear facet ratio, which is measured at the same level as the trochlear depth, compares the tangential length of the medial facet to that of the lateral facet (Fig. 13.42). A ratio of less than 0.4 is abnormal [27].
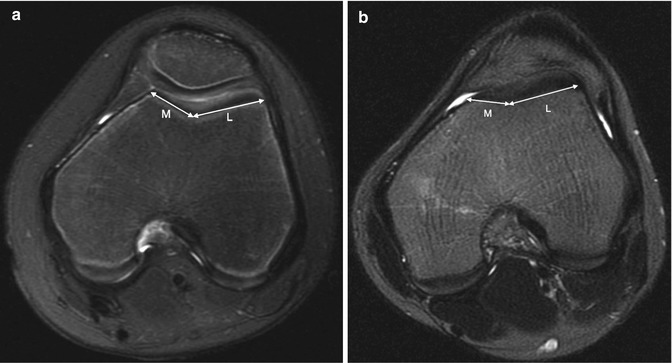

Fig. 13.42
Trochlear facet ratio. The transverse length of the medial trochlear facet compared to the lateral trochlear facet (M/L). (a) Normal. (b) Ten-year-old girl with abnormal facet ratio of 0.35 (axial T2-W FS MRI)
If the tibial tuberosity is too far lateral, the lateral force vector of the quadriceps femoris muscle at the patellofemoral articulation increases, contributing to patellar instability. The position of the tibial tuberosity in relation to the femoral trochlea is determined by measuring the horizontal distance between the midpoint of the anterior aspect of the tibial tuberosity and the deepest part of the trochlear groove (tibial tuberosity to trochlear groove (TT-TG) distance). The line through the trochlear groove is drawn perpendicular to a line drawn along the posterior femoral condyles. The line through the tibial tubercle then parallels the trochlear groove line. If the distance between these lines is less than 15 mm, the value is normal, 15–20 mm is borderline, and over 20 mm is abnormal (Fig. 13.43).
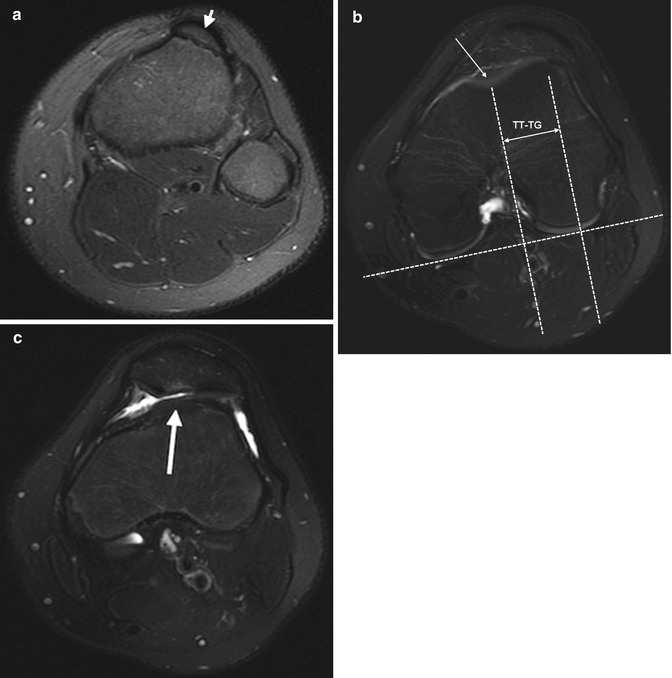

Fig. 13.43
Lateralization of the tibial tuberosity. The position of the tibial tuberosity relative to the femoral trochlea is assessed on axial images at two different levels: at the tibial tuberosity (a) and at the deepest point of the trochlea (b). The trochlear groove line is drawn through the deepest point of the trochlear groove (long arrow, b), perpendicular to a line tangent to the backs of the femoral condyle. The tibial tuberosity line is drawn through the midpoint of the tibial tuberosity (short arrow, a), parallel to the trochlear groove line. The distance between the parallel lines (double arrow, b) constitutes the tibial tuberosity-trochlear groove (TT-TG) distance. In this patient it measures 26 mm (normal less than 15 mm). (c) Axial T2-W FS image shows increased signal intensity in the patellar cartilage secondary to abnormal tracking, along with a small amount of joint fluid
If any of these measurements is abnormal, recurrent patellar dislocation is more likely (Fig. 13.44).
Patellar dislocation usually spontaneously reduces and is thus rarely encountered radiographically. If present, it is more apparent on the frontal view than the sunrise view (Fig. 13.45), as the flexed position partially reduces dislocation. The patella is also rarely dislocated at MRI, but slightly abnormal alignment may suggest previous or recurrent dislocation. The patella may be slightly laterally subluxated, and the medial aspect of the articulation may open, causing the medial portion of the joint to be wider than the lateral. In addition, “kissing contusions” may be evident. This marrow edema results from impaction during dislocation and is evident on the lateral aspect of the lateral femoral condyle as well as the medial aspect of the patella (Fig. 13.46). Articular cartilage injury on the medial patellar facet may be associated as well.
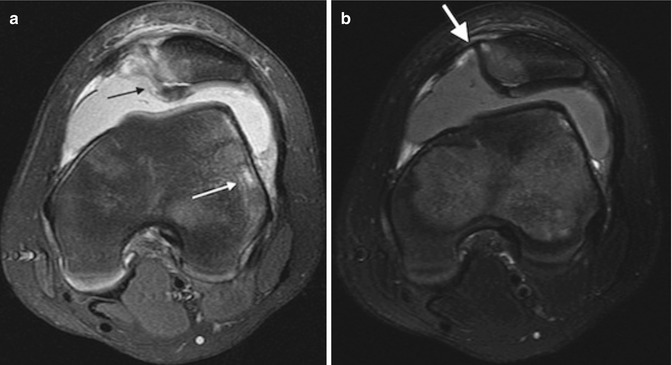

Fig. 13.45
Patellar dislocation in a 9-year-old girl with acute knee injury. (a) Frontal view of the right knee shows the patella is displaced laterally. (b) Sunrise view shows improved alignment with only mild lateral subluxation

Fig. 13.46
Recent traumatic patellar dislocation in a 12-year-old boy. (a) Axial T2-W FS image shows kissing contusion, with cartilage injury at the medial aspect of the patella (black arrow) and marrow edema at the lateral aspect of the lateral femoral condyle (white arrow). (b) Axial T2-W FS image shows focal marrow edema and fluid where the medial retinaculum attaches to the patella (white arrow)
The medial retinaculum may tear during dislocation and appear wavy or irregular (Fig. 13.47). This most frequently occurs at the patellar attachment in conjunction with an avulsion fracture of the patella’s medial cortex. However, tears can also occur in the mid-portion of the ligament or at the medial attachment of the retinaculum. With recurrent patellar dislocation, the retinaculum may be so attenuated that it cannot be identified.
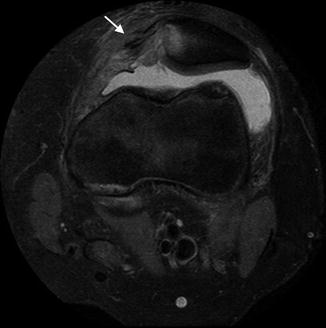

Fig. 13.47
Sequelae of recent traumatic transient patellar dislocation in a 15-year-old football player. Axial PD FS image shows tear of the medial retinaculum (arrow) as well as medial patellar marrow edema and cartilage injury (Courtesy of Sandra L. Wootton-Gorges)
3 Longitudinal Limb Deficiency (Hypoplasia and Aplasia)
3.1 General Considerations
Limb anomalies are encountered in the lower extremity about half as often as in the upper extremity [28]. Although patterns of malformation are generally similar in the upper and lower extremities, they differ in several interesting ways. In the upper extremity, if there is a significant abnormality in the proximal limb, the distal part of the extremity is almost certainly abnormal as well—for example, if the humerus is dysplastic, the radius and ulna are probably severely deformed. However, in the lower extremity, the femur may be markedly abnormal yet the tibia and fibula well formed or only slightly hypoplastic. In addition, the foot may be normal despite severe proximal anomalies—unlikely with the hand [29]. Another interesting difference is that although synostoses are relatively common in the forearm, they are very rare in the lower leg and in the lower extremity are essentially limited to the foot.
However, anomalies in some areas are often associated with nearby abnormalities, due to features of embryogenesis. For example, proximal focal femoral deficiency (PFFD) and fibular aplasia often occur together (i.e., postaxial limb bud deficiency). Some anomalies cluster as components of syndromes, whereas others are isolated. Proper limb development depends at least in part on sonic hedgehog gene clusters, and lack of these genes may contribute to severe truncation mutations [30].
As with the upper extremity, multiple classification systems have been devised (two are presented in detail in Chap. 7). These systems are generally either descriptive or based on treatment, and they can help guide therapeutic pathways as well as suggest prognosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree