Nadeem Parkar, Cylen Javidan-Nejad, Sanjeev Bhalla, Simon P.G. Padley
The Mediastinum, Including The Pericardium
The mediastinum is that anatomical region bounded laterally by the two lungs, anteriorly by the sternum, posteriorly by the vertebrae, superiorly by the thoracic inlet and inferiorly by the diaphragm.
Mediastinal Diseases
Mediastinal Masses
Incidence
The true prevalence of mediastinal masses is unknown as most surgical series are biased towards patients requiring surgery and do not include all aneurysms, intrathoracic goitres or lymph node masses in patients with established diagnoses such as lymphoma or sarcoidosis. In adult surgical series,1–3 the most frequent tumours are of neurogenic (17–23%), thymic (20–25%) or lymph node (10–20%) origin (usually neoplastic). Developmental cysts, thyroid masses and germ-cell tumours constitute the next most frequent group (approximately 10% each). In children, neuroblastoma/ganglioneuroma, foregut cysts and germ-cell tumours account for over three-quarters of cases, whereas thymoma and thyroid masses are rare.
The mediastinum is divided into compartments to aid in developing a differential diagnosis. However, there are no physical boundaries between compartments. Anatomically the mediastinum is divided into superior and inferior compartments by an imaginary line traversing the manubriosternal joint and the lower margin of T4 vertebra. The inferior compartment is further divided into three parts: anterior, middle and posterior. The Felson method of division is based on lateral radiography. A line that extends from the thoracic inlet to the diaphragm along the posterior cardiac surface and anterior to the trachea separates the anterior from middle compartments. The middle and posterior compartments of the mediastinum are separated by a line that runs 1 cm behind the anterior margins of the vertebral bodies. A popular modification divides the entire mediastinum into anterior, middle and posterior compartments but does not have a separate superior compartment.4 We find this latter approach helpful in our adult population.
Imaging Techniques
Mediastinal masses are often incidentally detected on chest radiograph. Despite diagnostic limitations, the chest X-ray (CXR) is important for detecting and localising mediastinal masses when suspected clinically.
Computed Tomography
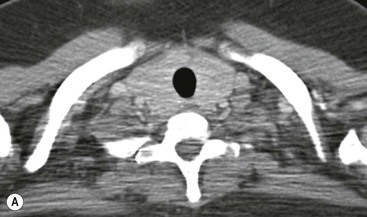
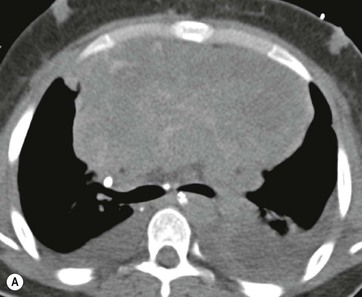
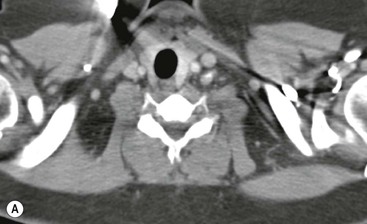
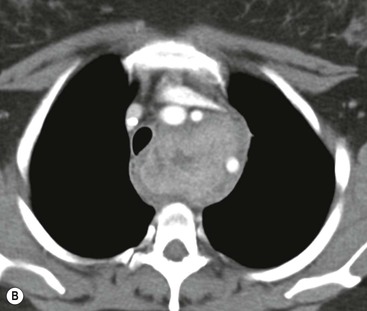
CT is the most useful investigation for localising, characterising and demonstrating the extent of a mediastinal mass and its relationships. CT is also useful in delineating calcification, which can help generate a more refined differential diagnosis. Multidetector CT (MDCT) following intravenous contrast medium with multiplanar reformats provides an excellent assessment of mediastinal structures, including vessels in the coronal and sagittal planes (Fig. 11-1). CT may guide biopsy, plan resection and follow response to therapy.
Magnetic Resonance Imaging
Magnetic resonance (MR) remains useful for imaging suspected neurogenic tumours, demonstrating intraspinal extension of a mediastinal mass and further evaluating the relationship of a mass to the heart, pericardium and larger intrathoracic vessels. MR may have advantages over contrast medium-enhanced CT for distinguishing between solid tissue and adjacent vessels (fast-flowing blood in vessels results in a signal void on spin-echo sequences) and may be useful for confirming that a mass is cystic. Unlike CT, MR is not as sensitive to the presence of calcification.
Ultrasound
Ultrasound of the mediastinum, including echocardiography and endoscopic ultrasound, may be of use in selected patients, in particular for distinguishing cystic from solid mediastinal masses and for distinguishing cardiac from paracardiac masses. Ultrasound is increasingly being used to guide mediastinal biopsy.
Radionuclide Examinations
Radionuclide examinations have a limited role in assessing mediastinal masses. Positron emission tomography (PET) and PET–CT using [F-18]2-deoxy-d-glucose (18F-FDG) has proven useful in evaluating mediastinal lymph node involvement in lung cancer and lymphoma.5,6 Radionuclide examinations may also be useful in imaging thyroid masses, neuroendocrine tumours and pheochromocytomas.
Approach to Mediastinal Masses
Localise to the Mediastinum
The CXR is the first step in evaluating mediastinal diseases. Findings that help in localising the lesion to the mediastinum include:
Localise within the Mediastinum
The information obtained from the relationship of the normal anatomical structures representing the ‘mediastinal lines and stripes’ on chest radiography aid in localising the lesion within the mediastinum to the anterior, middle or posterior mediastinum and generating a differential diagnosis before proceeding to a chest CT examination.7
Anterior mediastinal masses can be identified when both the hilum overlay sign and preservation of the posterior mediastinal lines are present. Widening of the right paratracheal stripe and convexity relative to the AP window reflection both indicate abnormality in the middle mediastinum. Disruption of the azygo-oesophageal recess can be caused by disease in either the middle or posterior mediastinum. Paravertebral masses disrupt the paraspinal lines, and the region of masses above the level clavicles can be inferred by their lateral margins: Posterior masses have sharp margins caused by their interface with lung, whereas anterior masses do not.8
Although there is no tissue plane separating the divisions of the mediastinum, attempting to localise an abnormality within the mediastinum helps in narrowing the differential diagnosis and determining appropriate further imaging.
Characterise on CT or MR
Mediastinal masses can be further characterised by CT or MR, depending on whether they contain fat, fluid or are vascular (Table 11-1).
Thyroid Masses
Most thyroid goitres are in the neck, yet between 3 and 17% of goitres extend into the thorax.9,10
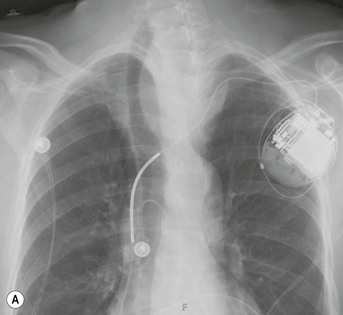
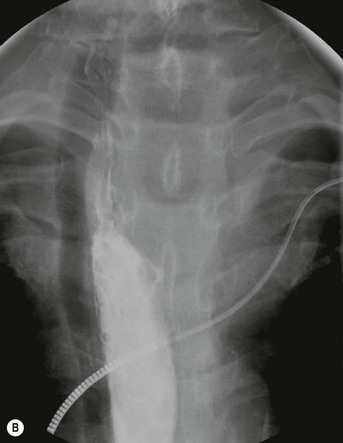
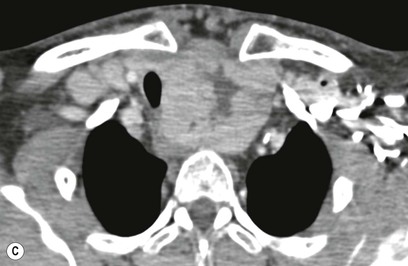
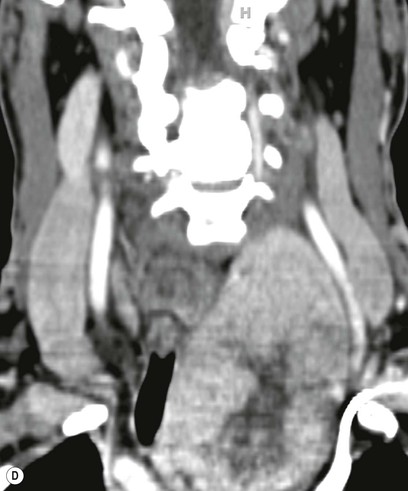
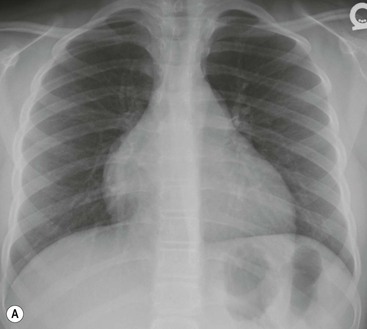
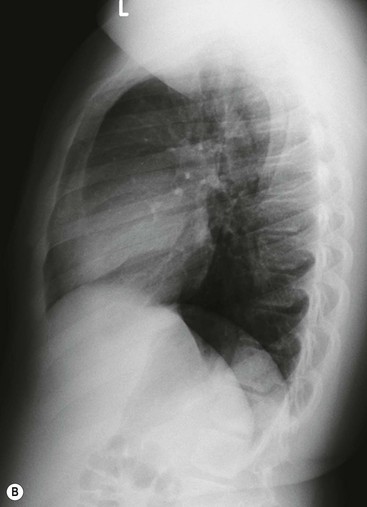
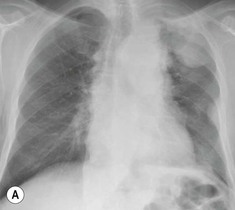
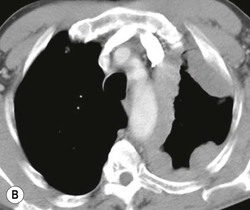
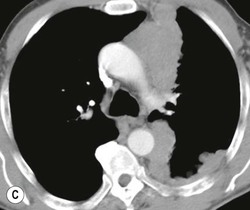
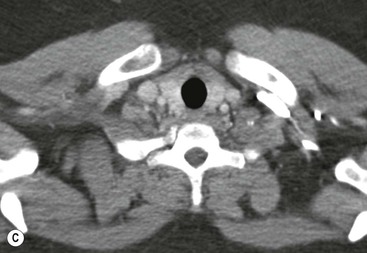
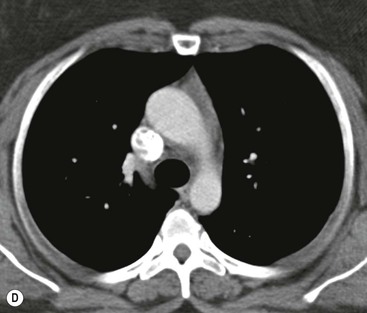
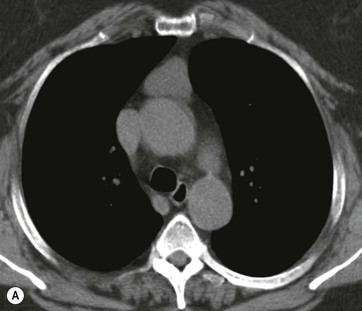
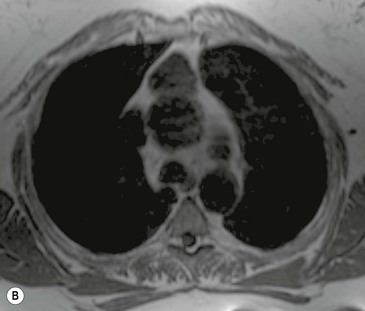
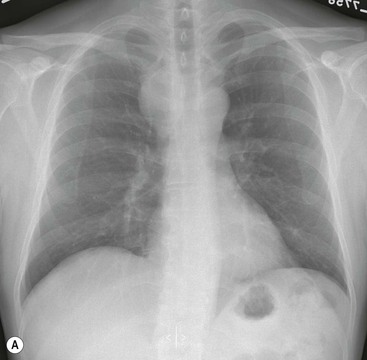
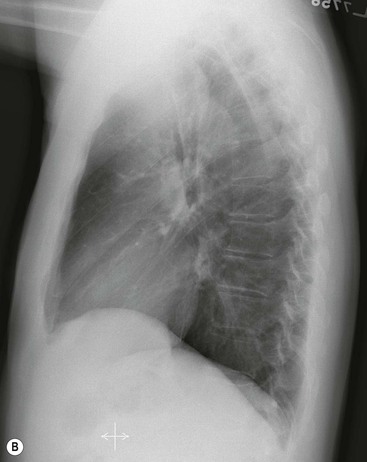
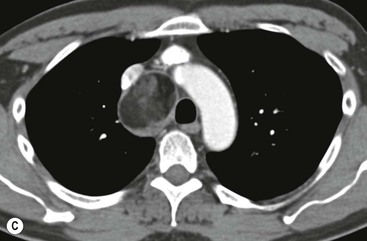
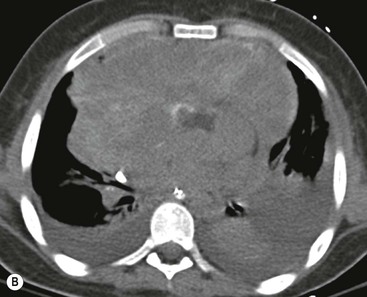
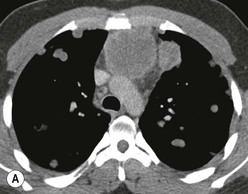
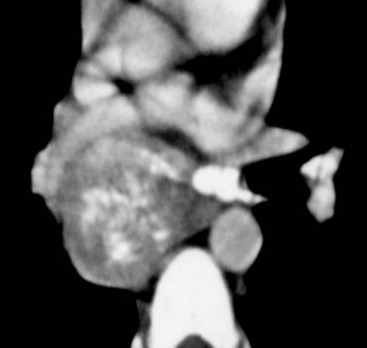
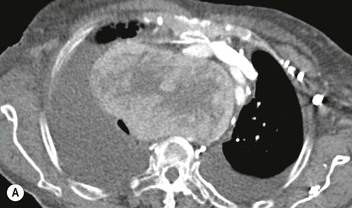
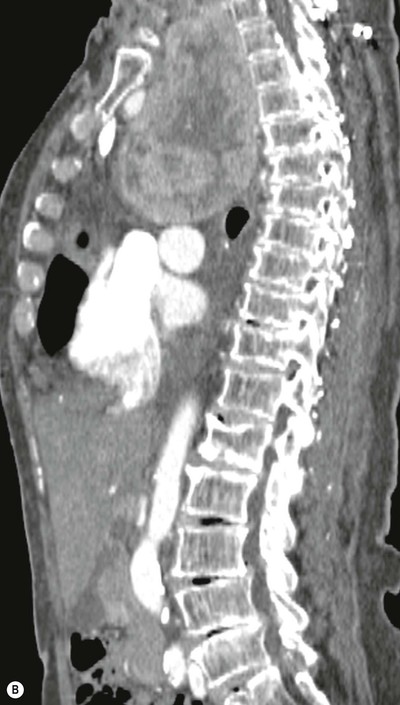
Most thyroid masses in the mediastinum represent downward extensions of either a multinodular colloid goitre or, occasionally, an adenoma or carcinoma. Intrathoracic thyroid masses usually have a well-defined spherical or lobular outline (Fig. 11-1). Rounded or irregular, well-defined areas of calcification may be seen in benign areas, whereas amorphous cloud-like calcification is occasionally seen within carcinomas.
Almost all intrathoracic thyroid masses displace the trachea and may cause tracheal narrowing. The direction of displacement depends on the location of the mass. Thyroid masses are most commonly anterior and lateral to the trachea. Posteriorly, masses often separate the trachea and the oesophagus, and such separation by a localised mass rising into the neck is virtually diagnostic of a thyroid mass.
CT imaging features of mediastinal thyroid goitres are:
1. Continuity of the mass with the cervical thyroid gland;
2. Foci of heterogeneous attenuation (cystic areas and calcifications);
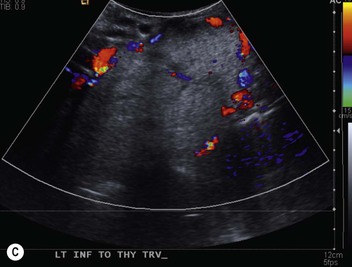
The most important of these features is to demonstrate continuity of the mass with the cervical thyroid. It is usually possible to diagnose a thyroid origin by noting a well-defined mass in the paratracheal or retrotracheal region, almost invariably being continuous with the thyroid gland in the neck. It is not possible to distinguish between a benign and malignant mass on CT unless the tumour has clearly spread beyond the thyroid gland. It should, however, be noted that multiple masses are a feature of benign multinodular goitre, though carcinoma can develop in multinodular goitre. MRI of intrathoracic goitre, like CT, can identify cystic and solid components, and in addition can demonstrate haemorrhage. In most practices, evaluation of the questionable thyroid usually relies on ultrasound with biopsy.
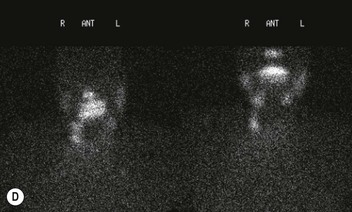
Radionuclide imaging with 123I or 131I demonstrates the presence of thyroid tissue within the mediastinum in almost all intrathoracic goitres. Although radionuclide imaging is a sensitive and specific method of determining the thyroid nature of an intrathoracic mass, CT is more useful as the initial investigation because it provides more information should the mass prove to be something other than a thyroid lesion and is almost as specific as nuclear medicine in diagnosing a thyroid origin. CT optimally demonstrates the shape, size and position of the mass11,12 (Fig. 11-2).
Parathyroid Masses
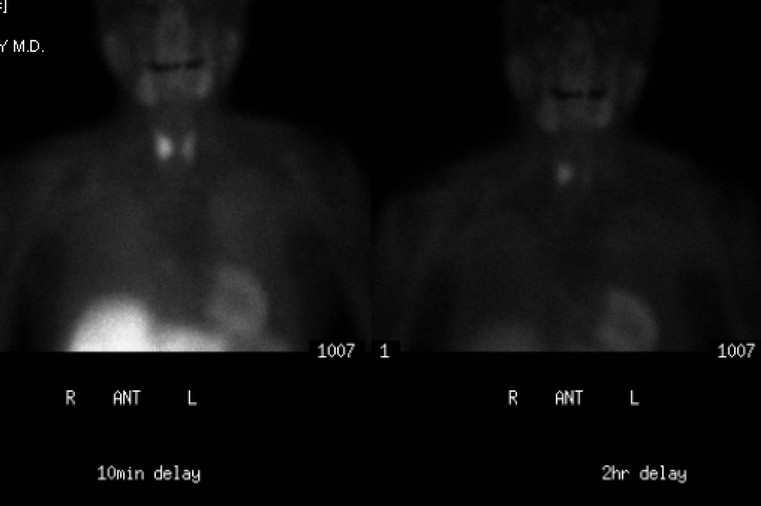
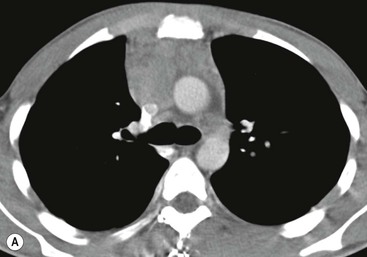
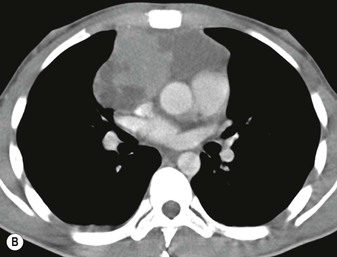
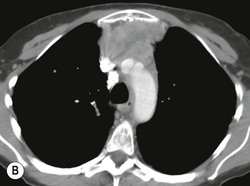
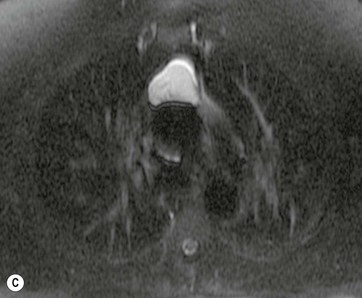
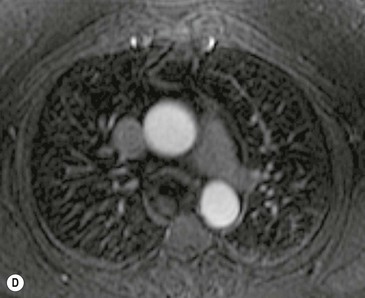
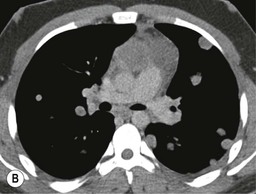
A parathyroid tumour is a rare cause of an anterior mediastinal mass. The parathyroid glands may migrate into the chest during fetal development. Mediastinal parathyroid tumours causing hyperparathyroidism are most commonly located in or around the thymus. In most patients with hyperparathyroidism, the aetiology lies within the parathyroid gland (either hyperplasia or a small adenoma) and escapes visualisation on CT. The role of CT or MR is for detection of ectopic adenoma, which is suspected when no lesion is detected by ultrasound or the hyperparathyroidism persists despite parathyroidectomy. The adenomas tend to be small and enhance homogeneously. Often, the CT is used in conjunction with 99mTc-sestamibi imaging13,14 (Fig. 11-3).
Thymic Tumours
The thymus is a bilobed, triangular-shaped organ that occupies the retrosternal space and varies widely in size and shape depending on the age.15 In subjects aged over 25, the thymus is no longer discretely recognised but seen as islands of soft-tissue attenuation within a background of fat. Although the thymus may still be seen as a discrete structure in adulthood, it usually involutes and is completely replaced by fat. Anterior mediastinal masses of thymic origin include thymomas, thymic carcinoma, thymolipoma, thymic lymphoma, thymic carcinoid and thymic hyperplasia. Thymic cysts may be simple cysts and occur in an otherwise normal gland, may lie within a thymoma or may follow thymic irradiation for Hodgkin’s disease.16
Thymomas
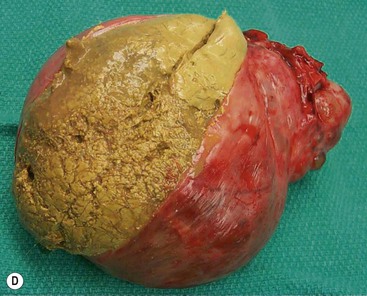
Thymomas are the most common tumour of the thymus in adults, and the most common primary tumour of the anterior mediastinum in adults.17 Thymomas are usually low-grade malignant tumours of thymic epithelium. Thymomas may be encapsulated (non-invasive thymomas) or may extend beyond the capsule (invasive type). The average age at diagnosis is approximately 50 years, earlier in those who present with myasthenia gravis. Thymomas are extremely unusual below the age of 15 and rare under 20. Up to 50% of patients with thymoma have myasthenia gravis, and approximately 10–20% of patients with myasthenia gravis have a thymoma. A variety of other syndromes are seen in patients with thymoma, including hypogammaglobulinaemia and pure red cell aplasia.18,19
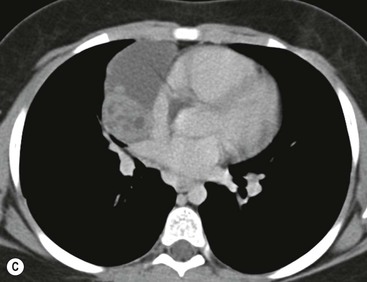
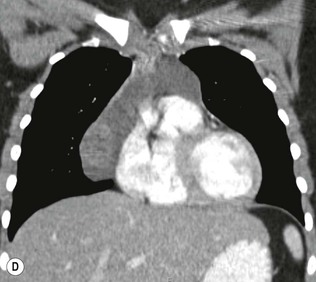
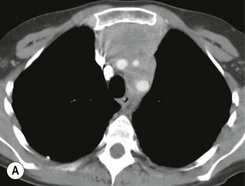
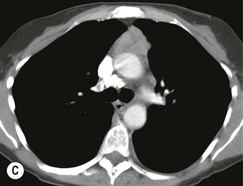
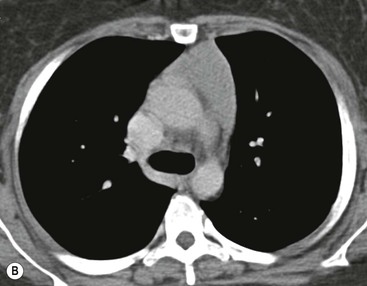
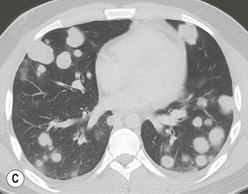
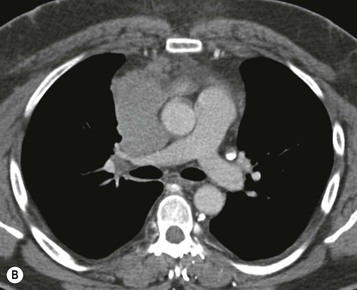
Most thymomas (90%) arise in the upper anterior mediastinum. The mass is usually anterior to the ascending aorta, lying above the right ventricular outflow tract and pulmonary artery. A few are situated more inferiorly, projecting from the left or right heart border, or lying close to the cardiophrenic angles (Fig. 11-4). They are usually spherical or oval in shape and may show lobulated borders. Thymomas may contain one or more cysts and a few are predominantly cystic. Calcification, punctate or curvilinear, may be seen. All of these features are best demonstrated using CT,20,21 which is the most sensitive technique for detecting thymoma in patients with myasthenia gravis.22 Thymomas as small as 1.5–2.0 cm in diameter are readily identified in men and women over the age of 40, largely because the rest of the thymus is atropic. Diagnosing a small thymoma in those patients younger than age 40, and particularly younger than 30, can be difficult, because the normal gland is variable in size and in myasthenia gravis the associated hyperplasia may cause a bulky gland. Fortunately, thymoma is so infrequent in children that the potentially difficult problem of finding a thymoma in a child with myasthenia gravis rarely arises.
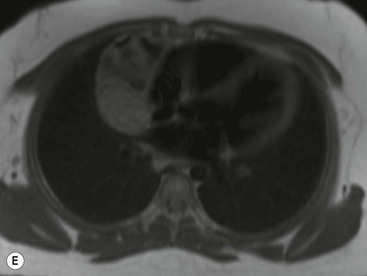
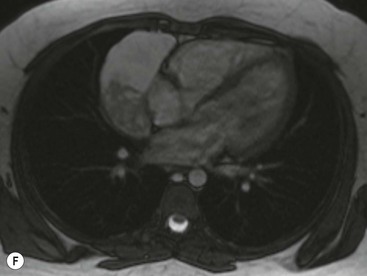
Thymomas usually show homogeneous density and uniform enhancement after contrast media injection and may occasionally be cystic. Invasion of the mediastinal fat and adjacent pleura may be identified with invasive thymomas, and while CT shows such invasion to advantage, it cannot reliably diagnose invasive thymoma if the tumour is still confined to the thymus.23 Pleural metastases resulting from transpleural spread are a feature of invasive thymomas, and therefore the whole of the pleural cavity should be carefully examined.23 On MR, the normal thymus in children and young adults characteristically demonstrates homogeneous and intermediate T1- and T2-weighted signal intensity, less intense than mediastinal fat but greater than muscle. In older patients after puberty, the T1 and T2 signals increase with age because the thymus begins to involute and is replaced by fat.24,25 Thymomas typically demonstrate low T1 signal intensity similar to muscle and relatively high T2 signal intensity. MR can be useful for showing mediastinal spread when there is doubt about the CT. T2-weighted images occasionally show lobulated internal architecture and scattered areas of high intensity that correspond to fibrous septa and cystic areas26 (Fig. 11-4). Heterogeneity of signal intensity caused by septation, cystic change and haemorrhage is common.
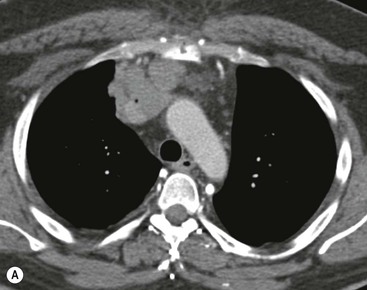
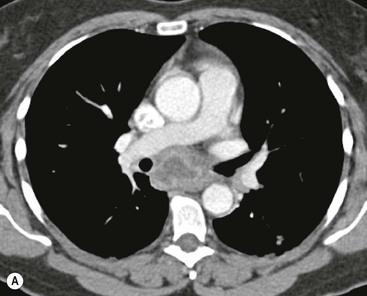
Invasive thymomas invade beyond the capsule into the mediastinum and can involve the pleura and pericardium. Complete obliteration of the adjacent fat planes suggests mediastinal invasion (Fig. 11-5). On the contrary, complete preservation of the adjacent fat planes excludes extensive invasive disease but does not rule out minimal capsular invasion.22 MR is superior to CT for defining the invasion of contiguous structures such as the pleura and pericardium in patients with invasive thymoma, but is not used in most cases.27
Thymic Carcinoma
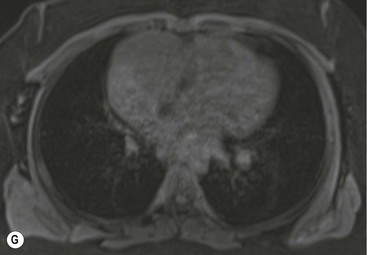
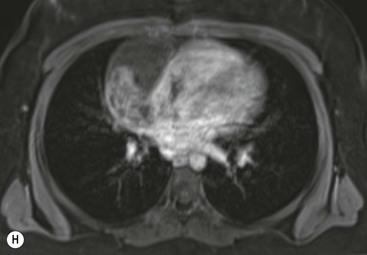
Thymic carcinoma is a thymic epithelial tumour with a high degree of anaplasia, cell atypia and increased proliferation28 and predominantly occurs in adults. Thymic carcinomas have a poor prognosis despite treatment with surgery and radiotherapy. Thymic carcinomas are aggressive, locally invasive malignancies that have frequently metastasised to regional lymph nodes and distant sites at presentation. They are typically large, heterogeneous masses, containing areas of necrosis and calcification, often demonstrating evidence of invasion of adjacent structures, in particular the mediastinum, pericardium and pleura29,30 (Fig. 11-6). On MR, thymic carcinoma demonstrates intermediate signal intensity slightly higher than muscle on T1-weighted sequence and high signal intensity on T2-weighted sequence.31 Thymic carcinomas are more commonly associated with mediastinal node and extrathoracic metastasis but less commonly associated with pleural implants compared with invasive thymomas.30
Thymic Neuroendocrine Tumour (Thymic Carcinoid)
Primary neuroendocrine tumours (carcinoids) of the thymus are rare and account for less than 5% of anterior mediastinal tumours. Unlike pulmonary carcinoids, these tumours are aggressive and at least 20% of patients have distant metastasis at presentation to the liver, lung, bone, pleura and pancreas.32 Approximately 40% of patients have Cushing’s syndrome because of adrenocorticotrophic hormone secretion by the tumour and up to 20% have multiple endocrine neoplasia (MEN) syndromes I and II.
Thymic carcinoid is histologically distinct from thymoma, although their imaging features overlap. On CT or MR, the tumours appear as a lobulated thymic mass with heterogeneous enhancement (Fig. 11-7) and central areas of low attenuation secondary to necrosis or haemorrhage and may show local invasion. Bone metastasis is typically osteoblastic.
Thymolipomas
Thymolipomas are rare tumours composed of a mixture of mature fat and normal-looking or involuted thymic tissue. The reported age range is 3–60 years. The average age of the patient is 22–26 years and most patients are asymptomatic.33 These tumours occur low in the anterior mediastinum, often in the cardiophrenic angle. Individual cases have been reported in association with a variety of conditions, including myasthenia gravis, aplastic anaemia, Graves’ disease and hypogammaglobulinaemia. Thymolipomas can grow to a very large size before discovery and, being soft, mould themselves to the adjacent mediastinum and diaphragm, and may mimic cardiomegaly or lobar collapse.34 CT shows the fatty nature of the mass, with islands of thymus and fibrous septa running through the lesion.33,34 On MR, fat within the tumour appears as high signal intensity and soft tissue appears as low signal intensity bands coursing through the mass.35
Lymphofollicular Thymic Hyperplasia and Rebound Thymic Hyperplasia
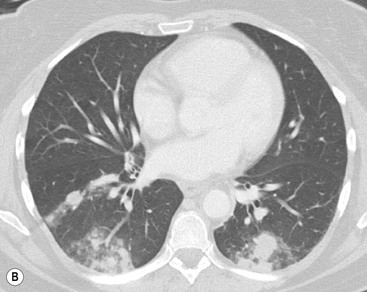
The most common association of lymphofollicular thymic hyperplasia is myasthenia gravis (seen in 50% of patients with myasthenia gravis), but lymphofollicular thymic hyperplasia is also seen in other conditions, such as thyrotoxicosis, systemic lupus erythematosus, Hashimoto’s thyroiditis and Addison’s disease. Thymic hyperplasia is rarely severe enough to cause visible enlargement of the thymus. When it does enlarge the thymus, both lobes are enlarged, usually uniformly (Fig. 11-8). Only rarely, hyperplasia may mimic a thymic mass.
The thymus may atrophy because of stress or as a consequence of steroid or antineoplastic drug therapy.36,37 The gland usually returns to its original size on recovery or cessation of treatment, but it may become larger than its original size (rebound thymic hyperplasia). Such rebound hyperplasia may be difficult to distinguish from neoplastic involvement. The diagnosis depends on a known reason for thymic rebound, the absence of clinical features to indicate tumour recurrence and the presence of an enlarged, normally shaped thymus.37,38 In patients over 15 with an enlarged thymus, chemical shift MR and fat-suppressed T2-weighted or short tau inversion recovery (STIR) imaging can diagnose thymic hyperplasia by detecting fatty infiltration or fat in the thymus, helping to differentiate from a neoplastic process. In thymic hyperplasia there is a drop in signal intensity at opposed phase images, while in thymic tumours there is no such reduction in signal. Chemical shift MR can depict physiological fatty infiltration of the thymus in 50% of persons aged between 11 and 15, in 100% of those >15 but in none of those <15 years.39,40
Thymic Cyst
Thymic cysts are uncommon, representing 1% of all mediastinal masses. They can be congenital or acquired. Congenital thymic cysts are derived from a patent thymopharyngeal duct and are usually unilocular. Approximately 50% of thymic cysts are discovered in those < 20 years. Acquired thymic cysts are multilocular and occur in association with thymic tumours, Langerhans cell histiocytosis or radiation therapy for Hodgkin’s disease. On chest radiographs, thymic cysts are indistinguishable from other thymic masses. On CT, simple congenital thymic cysts are seen as well-defined water attenuation masses with imperceptible walls. Multilocular thymic cysts appear as well-defined heterogeneous masses with imperceptible walls. On MR, thymic cysts demonstrate typical characteristics of fluid with low T1 and high T2 signal intensity (Fig. 11-9). If haemorrhage or infection occurs, then the cysts can demonstrate high signal on both T1- and T2-weighted images.
Germ-Cell Tumours of the Mediastinum
Germ-cell tumours account for 10–15% of anterior mediastinal masses in adults and approximately 25% in children. Germ-cell tumours of the mediastinum are believed to be derived from primitive germ-cell elements left behind after embryonal cell migration. The mediastinum is the most common extragonadal site for these tumours, approximately 60% of which arise in the anterior mediastinum. Most germ-cell tumours present during the second to fourth decades of life. Mediastinal germ-cell tumours include teratoma and a number of malignant forms, chiefly seminoma and non-seminomatous germ-cell tumours such as embryonal carcinoma, choriocarcinoma, endodermal sinus tumour and tumours with mixtures of these cell types.41 Malignant germ-cell tumours secrete human chorionic gonadotrophin and α-fetoprotein, which can be used as markers to diagnose and monitor the tumour. Malignant germ-cell tumours are almost always seen in male patients.
Teratomas
Teratomas contain elements of all three germinal layers: ectoderm (skin, teeth, hair), mesoderm (bone, cartilage, muscle) and endoderm (bronchial or GI epithelium). Teratomas usually have a benign course and surgical resection is the treatment of choice on the small chance that few malignant elements are present. Malignant teratomas have a poor prognosis. Teratomas are the most common mediastinal germ-cell tumour; most are cystic. Teratomas are found at all ages, particularly in adolescents and young adults, with women slightly outnumbering men.42,43 They are usually asymptomatic and diagnosed incidentally on chest radiography or CT, but may give rise to cough, dyspnoea or chest pain if they compress the bronchial tree or superior vena cava (SVC), or if they rupture into the mediastinum or lung. Teratomas are usually stable, but haemorrhage or infection may lead to a rapid increase in size.
On chest radiograph or CT most teratomas present as a well-defined, rounded or lobulated mass, localised to the anterior mediastinum. Fat and calcification may rarely be identified on chest radiograph. CT appearances are variable. Combinations of fat, fluid, soft-tissue components and calcification may be seen42 (Fig. 11-10).
MR is useful in differentiating teratoma from thymoma and lymphoma. Soft-tissue elements in the teratoma are isointense to muscle, cystic elements demonstrate low T1 intensity and high T2 intensity, and fat appears as high T1 intensity with signal loss on fat saturation sequence. Fat is virtually diagnostic of teratoma.44
Seminoma
Seminoma is the most common malignant mediastinal germ-cell tumour.45 Seminomas occur almost exclusively in men during the second through fourth decades. They are usually well-defined solid masses with possible small foci of degenerative changes representing haemorrhage and necrosis46 (Fig. 11-11). Symptoms are usually caused by mass effect on adjacent structures. On CT and MR seminomas have homogeneous attenuation and signal intensity and may have areas of haemorrhage and necrosis.
Non-Seminomatous Germ-Cell Tumours
Non-seminomatous germ-cell tumours include embryonal carcinoma, choriocarcinoma, endodermal sinus tumour and tumours with mixtures of these cell types. Malignant non-seminomatous germ-cell tumours are usually seen in young adults and are much more common in men (>90%) than in women. They are commonly more symptomatic than teratomas, from mass effect or invasion of adjacent structures.
The plain radiographic findings are similar, except that the malignant tumours are more often lobular in outline. Fat density and visible calcifications are rare. Because these are malignant tumours, they grow rapidly and metastasise readily to the lungs, bones or, less often, pleura (Fig. 11-12). CT often shows a lobular, asymmetric mass. The adjacent mediastinal fat planes may be obliterated, and the tumours either are of homogeneous soft-tissue density or show multiple areas of contrast media enhancement interspersed with rounded areas of decreased attenuation caused by necrosis and haemorrhage.47,48 On MR, these tumours may demonstrate heterogeneous intensities with areas of high T2 signal intensity corresponding to degenerative cystic changes.
Mediastinal Lymphadenopathy
Malignant Lymphoma and Leukaemia
Malignant lymphoma often involves mediastinal and hilar lymph nodes, multiple nodal groups usually being involved, particularly in Hodgkin’s disease. Any intrathoracic nodal group may be enlarged and the following generalisations regarding plain radiograph, CT and MRI findings can be made:
1. The prevascular, para-aortic and paratracheal nodes are the groups most frequently involved (Fig. 11-13). The tracheobronchial and subcarinal nodes also may be enlarged in many cases. In most cases, the lymphadenopathy is bilateral but asymmetric. Hodgkin’s disease, particularly the nodular sclerosing form, has a propensity to involve the prevascular, para-aortic and paratracheal nodes.
Lymph node enlargement is also seen occasionally with leukaemia, the pattern being the same as with lymphoma. The lymph node enlargement in both lymphoma and leukaemia may resolve remarkably rapidly with therapy.
Lymph Node Calcification
Extensive lymph node calcification is common following tuberculosis and fungal infection, and is occasionally seen with other infections (Fig. 11-14). It may also be encountered in a variety of other conditions, notably sarcoidosis, silicosis and amyloidosis. Although it may be seen in lymph node metastases from calcifying primary malignancies, such as osteosarcoma, chondrosarcoma and mucinous colorectal and ovarian tumours, lymph node calcification is rare in metastatic neoplasm. It is virtually unknown in untreated lymphoma though it is occasionally seen in nodes involved by Hodgkin’s disease following therapy.
CT is the most sensitive technique for the detection of lymph node calcification. Two common patterns of calcification are coarse, irregularly distributed clumps within the node and homogeneous calcification of the whole node. A strikingly foamy appearance is rarely seen with Pneumocystis jiroveci (previously Pneumocystis carinii) infection in AIDS patients49 and in some cases of metastatic mucinous neoplasms. Sometimes there is a ring of calcification at the periphery of the node—so-called ‘eggshell calcification’, which is a particular feature of sarcoidosis and of prolonged dust exposure (e.g. mining).
Low-Attenuation Nodes
On CT, areas of low attenuation within enlarged nodes, corresponding to necrosis, may be seen in a variety of conditions, particularly tuberculosis50 and occasionally in fungal disease,51 infections in immunocompromised patients, metastatic neoplasm (notably from testicular tumours52) and lymphoma53 (Fig. 11-15). Necrotic lymph nodes are common in patients with active tuberculosis. They demonstrate central areas of low attenuation with peripheral enhancement when intravenous contrast medium has been administered. Attenuation values below that of water are seen in fatty replacement of inflammatory nodes and have been described in Whipple’s disease.54
Enhancing Lymph Nodes
Castleman’s disease is a rare cause of strikingly uniform enhancing lymph nodes. Castleman’s disease (also referred to as angiofollicular lymph node hyperplasia) is a specific type of lymph node hyperplasia of uncertain aetiology which can cause substantial lymph node enlargement in many sites in the body. The lymph node mass is often localised to one area, can be huge and may be very vascular. The nodes may calcify and may show striking contrast media enhancement on both CT and MRI55,56 (Fig. 11-16). Histologically, there are two types: the hyaline vascular type and the plasma cell type.
In addition to Castleman’s disease, marked lymph node enhancement may occur in hypervascular metastases from melanoma, renal cell carcinoma, carcinoid tumours, papillary thyroid cancer and Kaposi’s sarcoma.57
Contrast media enhancement of enlarged nodes, when moderate in degree, is non-specific, being seen with inflammatory disorders, particularly tuberculosis, fungal disease, sarcoidosis and neoplasm. A low-density centre with rim enhancement of the enlarged node is a useful pointer towards the diagnosis of tuberculous or other granulomatous infections.
Lymph Node Enlargement
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree