The Neonatal Chest
Ramesh S. Iyer, MD
LEARNING OBJECTIVES
1. Describe the normal courses of umbilical arterial and venous catheters.
2. Identify the normal catheter positions of venoarterial (V-A) and venovenous (V-V) ECMO systems.
3. Recognize differences in patient size (trachea) and catheter size when identifying endotracheal tube and PICC positions in neonates, respectively.
4. Identify the typical features of diffuse lung disease in neonates, including:
Bilateral diffuse granular opacities in surfactant deficiency disease and GBS pneumonia.
Pleural effusions as useful adjunct features (e.g., common in neonatal pneumonia, rare in surfactant deficiency).
Transient tachypnea of the newborn appearing as pulmonary edema.
Coarse opacities and hyperinflation in meconium aspiration syndrome.
5. Identify pulmonary interstitial emphysema in the intubated neonate.
6. Recognize the typical features of bronchopulmonary dysplasia.
INTRODUCTION
Chest radiography is the most commonly performed imaging study in neonates. Often, x-rays are acquired to assess position of support devices for babies in the neonatal intensive care unit (NICU) or detect complications from device placement or prolonged use. Radiographs are also obtained to evaluate cause for dyspnea in the newborn. Pulmonary disease may be broadly divided into medical (nonsurgical) and surgical etiologies. Surgical lesions are congenital spaceoccupying masses and are discussed in the next chapter. Nonsurgical causes of neonatal respiratory distress are discussed here.
NICU SUPPORT DEVICES
Umbilical arterial catheters
Umbilical arterial catheters (UACs) are routinely used in preterm infants for arterial blood sampling, continuous blood pressure monitoring, and access for parenteral nutrition and medication administration.1,2 They are generally used only during the first week of life. Contraindications for UAC placement include omphalocele, cord anomalies, and vascular compromise to the buttocks or lower extremities.3
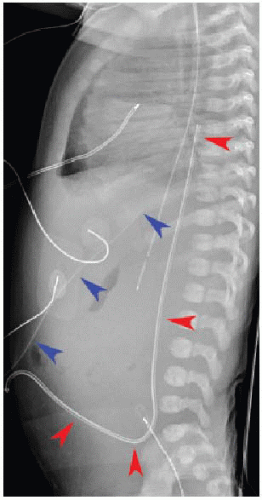
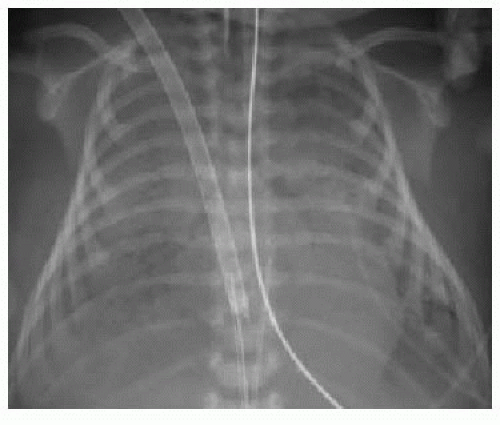
After passing through the umbilical artery, the normally positioned UAC traverses the internal iliac artery, common iliac artery, and aorta. On a frontal radiograph, the UAC courses inferiorly into the pelvis before entering the internal iliac artery and heading superiorly into the aorta along the left aspect of the spine (Fig. 3.1). On a lateral radiograph, the UAC is positioned posteriorly within the aorta just anterior to the spine (Fig. 3.2).2,4
Historically, two acceptable positions within the aorta have been described for UAC tips: “high” UACs terminating between T6 and T10 and “low” UACs terminating between L3 and L5.2,5 The reason was to avoid damaging the major abdominal aortic branch vessels.2 More recently, however, low UACs have been associated with higher rates of catheter dysfunction and complications.4,6, 7 and 8 At our institution, UACs are virtually always positioned in the descending thoracic aorta between T6 and T10 to ensure the catheter tip is below the level of the aortic arch, so as to preserve the major thoracic aortic branches supplying the neck and arms. Low-positioned UA catheters are preferred at other centers, however, and therefore, recognition of appropriate positioning below the level of the renal arteries is important. Arterial injury, embolization, and distal aortic thrombosis are potential complications with all UAC positions.4,6, 7 and 8
Umbilical venous catheters
Umbilical venous catheters (UVCs) are also frequently used in preterm infants for central venous pressure monitoring, blood gas measurements, and administration of fluid, antibiotics, and nutrition.9 Like UACs, these devices are generally used during the first week of life with similar contraindications to use.
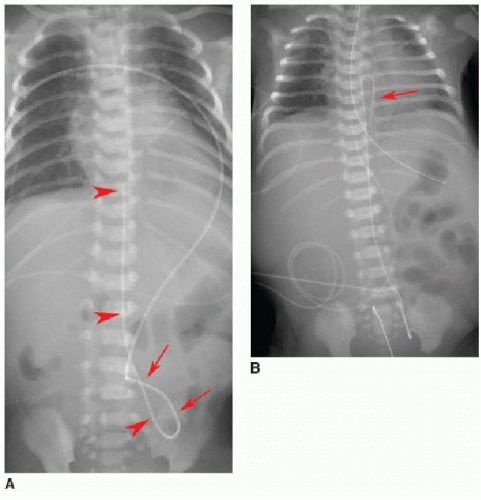
Normally positioned UVCs follow a cephalad course from the umbilical vein to the left portal vein, through the ductus venosus into the left hepatic vein, and terminate in the inferior vena cava (IVC) near its junction with the right atrium (RA).2,10,11 On a frontal radiograph, the UVC has a more direct path superiorly toward
the IVC-RA junction along the right of the spine (Fig. 3.3A), in contradistinction to the downward turn into the pelvis and ascent along the left of the spine seen with UACs. On a lateral projection, the UVC ascends superiorly and posteriorly from the umbilicus to the inferior vena cava, in contrast with the posteriorly positioned UAC (Fig. 3.2).10
the IVC-RA junction along the right of the spine (Fig. 3.3A), in contradistinction to the downward turn into the pelvis and ascent along the left of the spine seen with UACs. On a lateral projection, the UVC ascends superiorly and posteriorly from the umbilicus to the inferior vena cava, in contrast with the posteriorly positioned UAC (Fig. 3.2).10
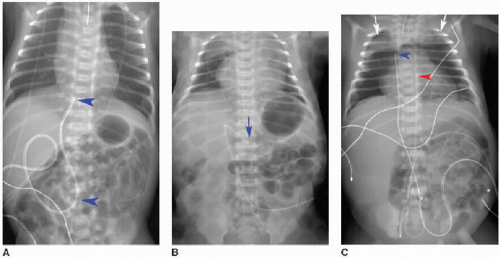
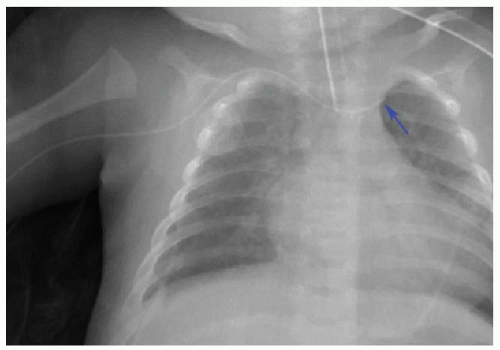
Malposition of the UVC may occur either above or below the IVC-RA junction. If advanced too far, the UVC enters the RA and potentially the left atrium via patent foramen ovale or atrial septal defect, a pulmonary vein or artery, or right ventricle through the tricuspid valve. This catheter may also be deflected at the level of the liver into either a left or right portal venous branch (Fig. 3.3B, C).10 Reported complications of UVC placement include portal hypertension and liver parenchymal injury and subsequent abscess formation.12,13
Extracorporeal membrane oxygenation catheters
Extracorporeal membrane oxygenation (ECMO) is the use of mechanical devices for survival in the context of cardiopulmonary failure. It provides time for diagnosis and treatment of the underlying condition. Patients considered for ECMO carry a high mortality risk, and the procedure may be viewed as a temporizing measure, or “last resort,” until effective medical treatment or organ transplantation ensues.14,15 In children, common indications for ECMO include meconium aspiration, congenital diaphragmatic hernia, surfactant deficiency, and sepsis.16 ECMO controls gas exchange and perfusion, providing physiologic stability.14 There are two general types of ECMO: venoarterial (V-A) for heart-lung support and venovenous (V-V) for respiratory support. Deoxygenated blood is removed from the RA, oxygenated through an artificial lung system, and delivered to the patient via either the aorta (V-A) or the RA (V-V).14,15
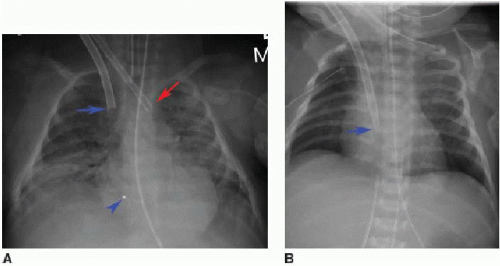
In venoarterial ECMO, catheters are introduced into the right internal jugular vein and the right common carotid artery. The venous catheter tip is placed into the RA, while the arterial catheter tip is positioned near the origin of the innominate artery (Fig. 3.4A). The carotid artery is frequently ligated cephalad to the catheter insertion site.16 In venovenous ECMO, a dual-lumen cannula is introduced into the right internal jugular vein, and normally the tip and side holes should be located in the RA (Fig. 3.4B).16, 17 and 18 In all ECMO systems, there are a variety of catheter appearances on radiography, and several catheters have distal portions that are radiolucent except for a radiopaque tip.18
Once ECMO begins, there is usually an increase in bilateral diffuse parenchymal opacity, creating a “whiteout lung” appearance (Fig. 3.5). This does not correlate with severity of lung injury, but is a phenomenon that likely reflects changes in pulmonary hemodynamics (edema) and abrupt decrease in airway pressure (atelectasis).18,19
The critical complication with ECMO is hemorrhage, a consequence of the system necessitating anticoagulation.14,15 Cranial sonography is routinely performed in patients on ECMO to monitor for intracranial hemorrhage, with CT providing a useful adjunct modality in this context.18,20 Thoracic complications of ECMO include catheter migration, air collections, pleural effusions or hemorrhage, and pulmonary hemorrhage.18
Endotracheal tubes
In older children, as in adults, it is common practice to report endotracheal tube position in terms of distance of the tip from the carina. For neonates this may be less helpful, given that the total tracheal length is frequently less than 4 cm, and there may be substantial movement of the tube with head position.21 Instead, it may be useful to comment on tube position in generic terms rather than numeric, such as termination “within the intrathoracic trachea” or “between the thoracic inlet and carina.” Direct communication should be made with the pediatric intensivist if the endotracheal tube has entered a main bronchus (frequently the right), remains above the thoracic inlet, or if there is concern for esophageal intubation.
Peripherally inserted central catheters
Peripherally inserted central catheters (PICCs) are frequently used in children for parenteral administration of fluid, antibiotics, and medications. These venous catheters may be introduced from either upper or lower extremity. Recent studies have documented increase in PICC insertions for hospitalized children, with concurrent decrease in catheter dwell time to reduce the risk of bloodstream infections.22,23 Radiologists should be aware of the small caliber of PICCs used in infants. These tubes are often between 2 and 4 French, or approximately 1 mm in diameter, which can render them challenging to identify on radiography.24 For upper extremity PICCs, the optimal position is the distal superior vena cava (SVC) near the cavoatrial junction. Lower extremity PICCs should similarly terminate in the IVC at or just below the IVC-RA junction.24, 25 and 26 PICC placement into noncentral veins, such as subclavian or brachiocephalic, is more prone to thrombosis, occlusion, and infection (Fig. 3.6). Conversely, PICCs entering the RA increase the risk of arrhythmia and rupture.24 Malpositioned PICCs should involve direct communication between the radiologist and intensivist so that rapid repositioning or removal may be facilitated.
DIFFUSE NEONATAL LUNG DISEASE
Surfactant deficiency disease
Surfactant deficiency disease (SDD) has also historically been referred to as hyaline membrane disease and respiratory distress syndrome (RDS). The condition primarily affects preterm infants ranging from 23 to 37 weeks of gestational age, and birth weights from 500 to 2,000 g. There is increased prevalence and disease severity in infants who are severely preterm (less than 30 weeks of gestational age) or of very low birth weight (less than 1,250 g).26,27 SDD is the most common cause of respiratory distress in preterm infants.28 The disease results from a deficiency of pulmonary surfactant in conjunction with structural immaturity of the lungs. Surfactant, produced by type 2 pneumocytes, reduces surface tension of the fluid lining of the lungs, thereby maintaining alveolar inflation.27,29 Immature lungs do not produce sufficient surfactant before 35 to 37 weeks of gestational age, resulting in alveolar collapse and poor gas exchange in preterm infants.27,




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree