The Normal Heart Scan: PET and SPECT
Philipp A. Kaufmann
This chapter provides a short summary of the commonly used tracers and techniques for assessing viability and perfusion by positron emission tomography (PET), along with examples of normal heart scans. The most commonly used tracer for clinical assessment of myocardial blood flow by PET is ammonia. The heart is displayed in short-axis sections beginning from the apex and ending at the base, as well as in vertical and horizontal long-axis sections. Liver accumulation of nitrogen 13 [13N]ammonia is often prominent. For viability, fluorine 18 fluorodeoxyglucose (18F-FDG) is actually the best-accepted PET tracer (see Fig. 28.2). The heart can be visualized by 18F-FDG only when the patient is not in a fasting state. Cardiac PET studies with this tracer are performed after a glucose load before scanning. Images are acquired 40 to 60 minutes after injection. For both ammonia and FDG, there is no tracer uptake into the membranous septum, very weak accumulation in the right ventricle, and frequently decreased uptake at the apex of the left ventricle.
Myocardial perfusion SPECT images look similar to myocardial perfusion PET images. Also, in the case of SPECT myocardial images, there is an increasing tendency to perform attenuation correction. This is facilitated by the new SPECT-CT imaging systems, which are currently gaining more and more acceptance.
Myocardial PET Perfusion Tracers
Various radionuclides are available for cardiac perfusion studies using PET (see Table 3.1). The positron-emitting radionuclides used in PET studies typically have short physical half-lives. These should be compared with the half-lives of radionuclides routinely used in diagnostic nuclear medicine, such as technetium 99m (99mTc) and thallium 201 (201Tl), which have half-lives of 6.0 and 73.5 hours. The short half-lives of positron-emitting radionuclides provide a number of practical advantages, in particular, the reduction of the radiation dose to the patient and the acquisition of repeated myocardial blood flow (MBF) measurements in the same patient in the same scanning session within a reasonable time (1 to 2 hours).
None of the tracers tested thus far have all the characteristics required for optimal quantification: short physical half-life, minimal radiation dose, uptake directly related to flow and independent of metabolic conditions, and availability without a cyclotron. At present, quantification requires sophisticated mathematical analysis, rapid data acquisition free of detector saturation, and, depending on the technique, concomitant administration of a blood pool tracer.
The three most common tracers are nitrogen 13 [13N]ammonia, rubidium 82 (82Rb), and oxygen 15 [15O]water; these tracers are discussed from a radiopharmaceutical point of view in Chapter 16. They have been selected for their physical and biochemical properties. Their physiologic characteristics show remarkable differences. [15O]water is metabolically inert and freely diffuses across all capillary membranes, including those of the myocardium, rapidly equilibrating between the vascular and extravascular spaces. There is only minimal effect of flow on [15O]water uptake and nearly 100% extraction (1). The 2-minute half-life of the 15O isotope allows repetitive MBF measurements at 10-minute intervals. However, [15O]water also remains in the blood pool, contaminating the image with uptake within the ventricular chambers
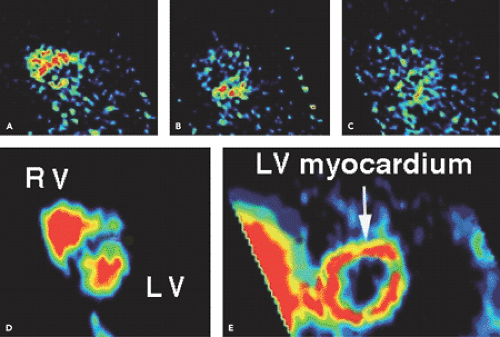
and surrounding structures. Therefore, a traditional shortcoming of the [15O]water technique used to be its need for additional [15O]carbon monoxide blood pool scans to define the regions of interest and correct for the high 15O activity in the blood pool. Recently, a new technique was proposed for generating myocardial images directly from the dynamic [15O]water scans, eliminating the need for additional carbon (C) 15O blood pool scans (2) while allowing serial [15O]water PET measurements of MBF (Fig. 28.1). This technique was validated against micro-spheres in experimental animals (3), and we have extensively documented its reproducibility in humans (4,5,6).
and surrounding structures. Therefore, a traditional shortcoming of the [15O]water technique used to be its need for additional [15O]carbon monoxide blood pool scans to define the regions of interest and correct for the high 15O activity in the blood pool. Recently, a new technique was proposed for generating myocardial images directly from the dynamic [15O]water scans, eliminating the need for additional carbon (C) 15O blood pool scans (2) while allowing serial [15O]water PET measurements of MBF (Fig. 28.1). This technique was validated against micro-spheres in experimental animals (3), and we have extensively documented its reproducibility in humans (4,5,6).
By contrast, [13N]ammonia provides high image contrast because it is rapidly cleared from blood and is avidly retained in myocardial tissue. Because of its tissue solubility, it diffuses readily into cells, where most is trapped as glutamate. At physiologic pH, ammonia is found primarily in the form of NH4+. Its concentration in the myocardium depends not only on flow but also on the amount of [13N]ammonia administered as a function of time (input function), its extraction at the instantaneous flow, and the metabolic state. The normal myocardial uptake pattern for PET using ammonia tagged with 13N is comparable to that obtained using fluorine 18 fluorodeoxyglucose (18F-FDG) (Fig. 28.2). However, it is obviously independent of the fasting state of the patient. There is often prominent liver accumulation of [13N]ammonia (Fig. 28.3).
The potassium analog 82Rb can be eluted from a strontium 82 generator system (see Chapter 16), avoiding the need for an on-site cyclotron. The extraction is flow related at high flow rates, and therefore a correction of 82Rb uptake for extraction is needed. The mean positron energy emitted by 82Rb is higher than that in [13N]ammonia. Thus the distance traversed by 82Rb positrons in tissue is longer, resulting in a somewhat lower spatial resolution. In addition to the lower count rate due to the shorter half-life, image quality is reduced compared with that obtained with [13N]ammonia. At present, the application of 82Rb must be considered immature because of these technical limitations, at least for the purpose of myocardial perfusion quantification, and it is very costly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree