Gynecologic imaging by ultrasound (US) has improved significantly during the past 20 years with the advent of various scanning techniques. The incorporation of transvaginal ultrasound (TVUS) has become a routine part of the gynecologic evaluation. Compared with transabdominal ultrasound (TAUS), tissue attenuation is usually less of an issue with TVUS because it allows the probe to be placed closer to the pelvic tissue, and higher frequency probes can be used. Recent advances in three-dimensional (3D) imaging have allowed the visualization of the coronal uterine plane and further improved the diagnostic accuracy of TVUS imaging.
Technical Requirements
The quality of a pelvic US examination is dictated by the correct selection of probes and the scanning experience of the sonologist or sonographer. The transducers and probes are characterized by their scanning area and their frequency. The scanning area is seen as rectangular with linear probes, whereas it is triangular with sector probes. The convex (curvilinear) probes are used most commonly, and the footprint will depend on their curvature. As the frequency of the probe is increased, the beam wavelength shortens and the resolution improves. The penetration decreases, however, with increasing frequency of the probe. Thus the highest frequency that has sufficient penetration should be used for optimizing the image quality. Two to 7 MHz for transabdominal and 5 to 12 MHz for transvaginal probes are used for pelvic scanning.
Safety
US is a form of energy. It has two major effects in tissues it traverses: heating and mechanical bioeffects. The American Institute of Ultrasound in Medicine (AIUM) guidelines conclude that there is no independently confirmed evidence to indicate damage in animal models below a thermal index (TI) of less than 2 and mechanical index (MI) of less than 0.3. The as low as reasonably achievable (ALARA) principle should be followed for all US examinations, but in particular for first trimester examinations and use of Doppler techniques.
Normal Anatomy
The uterus, tubes, and the ovaries are located in the pelvis. The pelvic brim is bordered by the sacral promontorium and linea terminalis formed by the iliac arcuate line, iliopectineal line, and the pubic crest. The pelvic brim separates the bony pelvis into two compartments: the greater or false pelvis and the lesser or true pelvis. The true pelvis is the compartment caudal to the pelvic brim. The greater or so-called false pelvis is the upper part of the pelvis above the pelvic brim and is occupied by bowel. An enlarged bladder or pelvic masses may also extend to the greater pelvis. TAUS may be more helpful in those cases because TVUS is mostly limited to the true pelvis.
General Anatomic Descriptions
Uterus and the Fallopian Tube
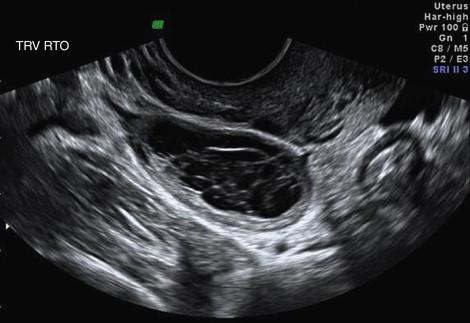
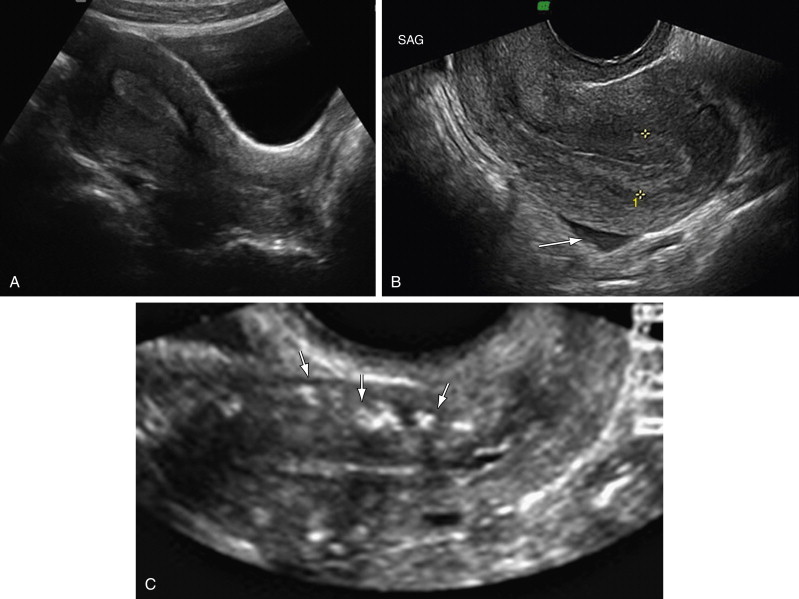
The uterus is located between the bladder and the rectosigmoid colon. The uterus has two parts: corpus or body and cervix. The junction of the corpus with the cervix is called the isthmus . The fallopian tubes originate from the cornua of the uterus. The upper part of the corpus above the fallopian tubes is called the fundus . The anterior lower portion of the uterus is continuous with posterior wall of the bladder separated by a connective tissue layer. The upper anterior, lateral, and posterior uterine walls are covered by the peritoneum. The peritoneal folds between the bladder and the rectum are called anterior cul-de-sac and posterior cul-de-sac or pouch of Douglas . A mild amount of free fluid in the cul-de-sac is a variant of the normal ( Figure 1-1 ) ; however, increased amount of fluid in the posterior cul-de-sac and any fluid in the anterior cul-de-sac usually indicates pathology.

The uterine size and shape are related to age and parity. In term neonates, the length of the uterus is correlated with birth weight and ranges from 2.3 to 4.6 cm, with a mean of 3.4 cm. The cervix is larger than the fundus (fundus-to-cervix ratio = 1:2), and the maximum thickness is approximately 1.4 cm; the endometrial lining is often echogenic. A small amount of fluid in the cavity can be seen secondary to high estrogen levels before delivery. The prepubertal uterus has a tubular configuration; however, in some cases the anteroposterior dimension of the cervix is larger than the anteroposterior dimension of the fundus, with a spade shape. The endometrium can be visualized as a thin echogenic line using high-frequency transducers. The length of the prepubertal uterus is 2.5 to 4 cm, and the anteroposterior dimension of the uterus does not usually exceed 10 mm.
The uterus starts to grow before menarche and continues to grow for several years.
The pubertal uterus has the adult pear configuration with the fundus of equal size or larger than the cervix and measures 5 to 8 cm long, 3 cm wide, and 1.5 cm thick. The fundus-to-cervix ratio is 1:1 to 3:2 in nulliparous women and 3:2 to 2:1 in multiparous women. The dimensions of the uterus in nulliparous and multiparous women are 6 to 8.5 cm and 8 to 10 cm in length, 3 to 5 cm and 4 to 6 cm in width, and 2 to 4 cm and 3 to 5 cm in the anteroposterior dimension, respectively. The uterine size decreases after menopause with a tendency of the fundus-to-cervix ratio to decrease.
The cervix is fixed in the pelvis by ligaments, whereas the uterus can assume various positions. In the majority, the uterus is anteverted, that is, tilted anteriorly, at the cervicovaginal junction and sits on the bladder. Version describes the relationship of the cervix to the vagina. Flexion describes the relationship of the cervix to the uterus. A retroflexed uterus is tilted backward instead of forward at the junction of the cervix and body, that is, the isthmus. A retroverted uterus refers to a backward tilt of the entire uterus, including the cervix ( Figure 1-2 , A and B ).
The myometrium sometimes appears to have three zones, not necessarily well demarcated. The inner layer appears hypoechoic and thin. It is not always seen clearly. The middle layer appears homogenous and is the muscle layer arranged in spiral configuration. The outer layer is thin and separated from the middle layer by arcuate vessels, which are variably evident by US. Calcifications may be seen in the arcuate vessels of older women ( Figure 1-2 , C ).
The endometrium is composed of a basal layer and functional layer shed each month. The endometrial thickness is measured perpendicular to the longitudinal axis excluding the hypoechoic subendometrial area, that is, the inner myometrium, and should be measured at the thickest point (see Figure 1-2 , B ) including the thickness on each side of the endometrial cavity. If there is fluid in the endometrial cavity, the endometrium should be measured excluding the fluid rim ( Figure 1-3 ). The endometrium is poorly seen in the majority of the patients with a retroverted uterus on TAUS examination because of the angle and backward position of the fundus; TVUS can usually allow adequate endometrial evaluation in those cases. For a good quality endometrial thickness measurement, a well-defined distinct endometrial echo should be seen extending from the endocervical canal to the fundus (see Figure 1-2 , B ). When visualization is suboptimal as a result of fibroids, previous surgery, marked obesity, or an axial uterus, the endometrial echo should be reported as “poorly seen.” An axial uterus, also sometimes termed a midpositioned uterus, occurs when uterine long axis is in a partly retroverted position, such that the uterine long axis extends directly away from the probe. Saline infusion sonohysterography (SHG) and hysteroscopy are both appropriate next steps in the endometrial evaluation of such patients, if patients have a history of bleeding. The endometrial texture should be assessed, and if heterogeneous and irregular, this may be a more important determinant than endometrial thickness. Because endometrial carcinoma, polyps, and hyperplasia can be focal, the entire endometrium (from cornua to cornua) should be imaged in longitudinal and transverse views.

The endometrium varies in appearance during the menstrual cycle ( Figure 1-4 ). The endometrium appears ultrasonographically as a thin, hyperechogenic single line immediately after menses in the early proliferative phase of the menstrual cycle. The slightly hyperechoic, well-defined endometrium gradually thickens up to approximately 8 mm. The functional and basal layers can be visually differentiated during the mid–late follicular phase. In the late follicular and periovulatory period, the endometrium assumes a trilaminar appearance with a central echogenic line of opposing functional layers, which are hypoechoic, and slightly hyperechoic basal layers more peripherally, and may measure up to 12 to 16 mm. A homogeneous, hyperechoic endometrium is observed as endometrial glands branch and expand under the influence of luteal progesterone production in the secretory phase. If pregnancy occurs, echogenicity and thickness are maintained as decidual reaction to implantation starts to progress. If pregnancy does not occur, the endometrium begins to regress in thickness, with echogenicity remaining similar or becoming heterogenous, finally ending in breakdown of the functional layer. An occasional tiny echogenic focus may sometimes be seen near the interface of the endometrium and myometrium. These are probably of no clinical significance and may be due to dystrophic calcifications from previous instrumentation.

In postmenopausal women with vaginal bleeding, large prospective studies have shown that an endometrial thickness of 4 mm or less on TVUS has a small risk for malignancy of 1 in 917 cases. Thus biopsy is not indicated in postmenopausal patients with bleeding when endometrial thickness is 4 mm or less. In the postmenopausal patient without any bleeding, there is considerable disagreement for defining the upper limit of endometrial thickness, giving a range of 5 to 15 mm. The significance of a thick endometrial echo in nonbleeding postmenopausal women has not been validated in a prospective trial. Routine tissue sampling in this group is not recommended; however, a recent study in asymptomatic women with endometrial thickness of 6 mm or more reported a 3.1% risk for endometrial carcinoma using both hysteroscopy as well as dilation and curettage.
Screening for endometrial pathology in women receiving hormone replacement is not recommended. The endometrial thickness shows a wide range of variation in asymptomatic women receiving estrogen alone or in some combination of estrogen and progestin with a mean endometrial thickness of 6.0 mm (range, 1 to 15 mm).
Tamoxifen-induced subepithelial stromal hypertrophy leads to poor correlation between endometrial pathology and ultrasonographic endometrial thickness with low specificity and with positive predictive values as low as 1.4%.
The cervix should be demonstrated on both transabdominal and transvaginal images although it is best seen on transvaginal images. The cervical palmate folds or plicae palmatae are the mucosal folds in the cervical canal and can sometimes be visualized by TVUS. Blockage of endocervical glands results in a frequent finding of nabothian cysts ( Figure 1-5 ). Nabothian cysts are usually of no clinical significance. Tiny echogenic foci may sometimes be seen centrally in the cervix. They have been found in association with chronic cervicitis and are also speculated to be due to dystrophic calcifications from previous instrumentation. They generally are of no clinical significance.

The fallopian tubes, or oviducts, extend outward from the superolateral portion of the uterus and end by curling around the ovary. The fallopian tubes connect the cornua of the uterine cavity and the peritoneal cavity. The oviducts are between 10 and 14 cm in length and slightly less than 1 cm in external diameter. The mesentery of the tubes, the mesosalpinx, contains the blood supply and nerves. Each tube is divided into four anatomic sections: The intramural or interstitial segment is 1 to 2 cm in length and is surrounded by myometrium. The isthmic segment begins as the tube exits the uterus and is approximately 4 cm in length. This segment is narrow, 1 to 2 mm in inside diameter, is straight, and has the most highly developed musculature. The ampullary segment is 4 to 6 cm in length and approximately 6 mm in inside diameter. It is wider and more tortuous in its course than other segments. Fertilization normally occurs in the ampullary portion of the tube. The infundibulum is the distal trumpet-shaped portion of the oviduct. Approximately 20 to 25 irregular finger-like projections, termed fimbriae, surround the abdominal ostia of the tube. One of the largest fimbriae is long enough to reach the ovary, the fimbria ovarica .
The interstitial portion of the fallopian tube may be seen on transverse uterine sections and on coronal 3D imaging. The other parts of the fallopian tubes are not usually visualized unless there is a pathologic process that enlarges the tube or in the presence of significant amount of fluid in the adnexal region ( Figure 1-6 ).

Embryologic Remnants
Hydatids of Morgagni are common simple cysts that occur near the fimbriated ends of the fallopian tube. They are Wolffian duct remnants and can achieve the size of approximately 1 cm. They can rarely undergo torsion with infarction by strangulation of their mesentery. Paraovarian cysts may be Wolffian duct or paramesonephric duct remnants, with the latter occurring more commonly within the broad ligament rather than at the fimbriated ends of the fallopian tube. Paraovarian cysts are usually simple cysts that range from 1 to 8 cm, and are usually asymptomatic but can infrequently become symptomatic as a result of enlargement and/or torsion. Sonographically, one cannot generally distinguish hydatids of Morgagni from paraovarian cysts, and the distinction is usually clinically insignificant.
The Ovaries
The ovaries are a pair of oval-shaped glands, usually located lateral to the uterus and medial to the internal iliac vessels in the ovarian fossa. Their position can vary within the pelvis, however. The ovarian volume can be assessed by the formula for ellipsoids: height × width × depth × 0.52. The size of the ovaries varies depending on age and parity ( Figure 1-7 ). The ovarian volume among children up to 24 months old can be larger than 1 cm 3 , and small cysts or follicles may be observed. The mean volume is approximately 1.1 cm 3 among girls up to 1 year of age and decreases to 0.67 cm 3 among girls 13 to 24 months old. Cysts larger than 9 mm can be seen in 18% of the ovaries in girls aged 1 day to 12 months. The ovaries grow in size between the age of 2 and 14 years. The number of follicles larger than 5 mm increases from 7 to 9 years of age. During the reproductive years, ovaries measure approximately 1.5 × 2.5 × 4 cm. The average ovarian volume in premenopausal women is approximately 7.4 to 7.8 cm 3 (standard deviation [SD], 2.4 to 2.6) and is not influenced by parity. The ovarian volume decreases in the postmenopausal patient showing a significant relationship with the number of years postmenopause. Average ovarian volume in the early postmenopausal patient is 3.4 to 3.8 cm 3 (SD, 1.3 to 1.6) and in late menopause is 2.5 cm 3 (SD, 1.1 to 1.3).

Human follicular development occurs from a diameter of approximately 0.03 mm and continues for more than 150 days until ovulation is achieved. Follicles are visible by US at only relatively advanced stages of development (i.e., ≥4 mm). They grow in minor and major waves of development, with smaller follicles appearing to grow and regress in a random fashion during the interovulatory interval. The growth dynamics of follicles up to 4 mm in diameter are not known. During the menstrual cycle, several ovarian follicles grow to 8 to 12 mm in diameter. The dominant follicle can be recognized, usually at a mean size of 10 mm, at day 8 to 12 of the menstrual cycle. It starts to differ from other follicles and increases in size 2 to 3 mm/day. The intermediate follicles usually grow to less than 15 mm. The growth of the dominant follicle continues and at the time of ovulation has a mean diameter ranging from 17 to 27 mm. In a menstruating woman, a simple ovarian cyst up to 25 mm most likely represents a follicle and should not be reported as a cyst. Disappearance or sudden decrease in size of the dominant follicle and appearance of free fluid in the cul-de-sac are the most sensitive markers of follicle rupture and ovulation. Irregularity of the follicle walls and internal echoes may also occur with follicle rupture, although they appear to have lower sensitivity and specificity for follicle rupture. After release of the egg, the dominant follicle partially collapses, forming a corpus luteum. There may be some internal bleeding as a result of vascularization of the granulosa layer of the ovary after ovulation, forming a corpus hemorrhagicum. The corpus luteum atrophies to a corpus albicans that is not usually visible by US. When supporting a conceptus, the corpus luteum maintains its hormonal secretion during the first trimester. Its size remains static from 5 to 9 weeks’ gestation (mean, 17 mm) and gradually regresses with almost 20% undetectable by 10 to 13 weeks, when placental hormone production takes over.
Functional ovarian cysts are seen in reproductive women and fall into two categories: follicular and corpus luteum cysts. Follicular cysts arise when this physiologic release fails and follicular growth continues as a result of either excessive stimulation by follicle-stimulating hormone or from lack of the normal preovulatory luteinizing hormone surge. Follicular cysts rarely grow larger than 10 cm, and most are asymptomatic. Although central hemorrhage into the collapsed follicle after ovulation is normal, expansion of the cavity by hemorrhage is consistent with a hemorrhagic corpus luteum. At some size, the term hemorrhagic corpus luteum cyst may be appropriate, but that size is not clear. Hemorrhagic cysts can vary in size ranging from approximately 3 to 8.5 cm. The hemorrhagic cyst has enhanced through-transmission signifying the basic cystic nature of the mass, and has a wall of variable thickness. Fine reticular echoes representing fibrin strands with no blood flow on Doppler US are the most common appearance ( Figure 1-8 ). The cyst shows change in echo pattern with time, related to the temporal sequence of clot formation and lysis. Hemorrhagic ovarian cysts can be followed sonographically to spontaneous resolution in 6 to 8 weeks; most completely resolve in 6 weeks or decrease considerably in size and change in morphologic appearance. The various phases of the retraction of the blood clot can result in sonographic images that may mimic a fluid level or a papillary excrescence. Rupture of a hemorrhagic corpus luteum can occasionally happen and may mimic an ectopic pregnancy.