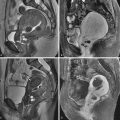
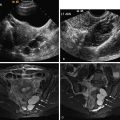
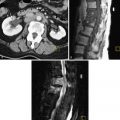
There are numerous causes of infertility. Tubal and uterine causes have been discussed in Chapters 17 and 18, respectively. The ovary may also be the source of infertility. The most common primary ovarian abnormality associated with infertility is polycystic ovary syndrome (PCOS) and will be the focus of this chapter. Other ovarian abnormalities, such as endometriomas, may be associated with infertility and are discussed in Chapter 29 .
Polycystic Ovary Syndrome
PCOS is the most common endocrine disorder of women of reproductive age, and the leading cause of anovulatory infertility among premenopausal women. The disorder is characterized by anovulation or infrequent ovulation, hyperandrogenism, obesity, and, in many cases, the presence of numerous small follicles on transvaginal ultrasound (TVUS) ( Figure 19-1 , A ). The prevalence of the disorder is 6% to 8% of women worldwide, and up to 12% in women with increasing body mass index (BMI), when the National Institute of Child Health and Human Development (NICHD) 1990 criteria for the disorder are applied.
Disease
Definition
(See discussion.)
Prevalence and Epidemiology
There is no consensus on the criteria used to establish the diagnosis of PCOS. Until recently, two definitions were accepted: the National Institutes of Health (NIH) conference criteria, and those suggested by the European Society of Human Reproduction and Embryology (ESHRE)/American Society for Reproductive Medicine (ASRM). The Androgen Excess and PCOS Society (AE-PCOS) has proposed a third definition. The AE-PCOS Society suggests there should be acceptance of the original NIH criteria with modification and acknowledges the opinions expressed in the proceedings of the 2003 Rotterdam conference; a cardinal feature of PCOS is ovarian morphology ( Figure 19-1 , B ). A principal conclusion of the AE-PCOS report, however, is that PCOS should first be considered a disorder of androgen excess or hyperandrogenism. The three definitions are summarized in Table 19-1 .
| NICHD/NIH/1990 | Rotterdam 2003 | AE-PCOS/2009 | |
|---|---|---|---|
| Diagnostic criteria |
|
|
|
| Exclusion criteria | Congenital adrenal hyperplasia, androgen secreting tumors, Cushing’s syndrome, and hyperprolactinemia | Congenital adrenal hyperplasia, androgen-secreting tumors, and Cushing’s syndrome | 21-Hydroxylase-deficient nonclassic adrenal hyperplasia, androgen-secreting neoplasms, androgenic–anabolic drug use or abuse, the hyperandrogenic-insulin resistance-acanthosis nigricans syndrome, thyroid dysfunction, and hyperprolactinemia |
| Clinical traits | Hirsutism, acne, and alopecia | Hirsutism, acne, and androgenic alopecia | Hirsutism |
| PCOM | Not included |
|
|
The establishment of the diagnosis of PCOS imparts substantial psychological, social, and economic consequences in the care of these women. The diagnosis implies an increased risk for type 2 diabetes mellitus, dyslipidemia, hypertension, endometrial adenocarcinoma, infertility, obesity, dysfunctional uterine bleeding, and possibly cardiovascular disease for these patients. Furthermore, the diagnosis may have important familial implications for the patient’s sisters and daughters. Careful selection of patients with PCOS would allow for the appropriate screening and treatment of women who are at significant risk for life-long sequela, without significantly adding to the overall health care burden. Furthermore, establishing the appropriate criteria for identifying women with PCOS would provide a guide for current and future research.
The 2003 ESHRE/ASRM-sponsored PCOS consensus group reemphasized the importance of polycystic ovarian morphology (PCOM) as determined by ultrasound (US) in the diagnostic criteria. The terms polycystic ovary (PCO) or polycystic ovaries (PCOs) are used synonymously for PCOM. PCO, PCOs, and PCOM all refer to the US appearance of the ovaries, not to the syndrome. PCOM or PCO alone does not indicate that the patient has PCOS. The inclusion of PCOM in the diagnostic criteria, however, sparked a controversy. Application of these criteria allowed for the inclusion of two new phenotypes in the spectrum of PCOS and expanded the potential number of women who would meet the diagnostic criteria. Patients with hirsutism and/or hyperandrogenemia with PCOM but who ovulate normally, and women with PCOM and irregular ovulation, but no signs of androgen excess could now be defined as having PCOS. Further adding to the controversy is the observation that although PCOM is found consistently in women with PCOS, a similar morphology is often seen in up to 25% of normal control subjects. The debate continues about the utilization of US and PCOM in the diagnosis of PCOS.
Etiology and Pathophysiology: Role of Genetic and Environmental Factors
Familial clustering of PCOS phenotypes and the associated metabolic and reproductive abnormalities suggests a role of genetic factors in the development of PCOS. The heterogeneity of phenotypic features within different families, and even within the same family, however, underscores the importance of the environmental contribution. It is now well established that PCOS represents a complex trait similar to type 2 diabetes and obesity and that both genetic and environmental factors contribute to the pathogenesis of PCOS. PCOS can be viewed as a heterogeneous androgen excess disorder with varying degrees of metabolic abnormalities. The precise mode of inheritance of PCOS has not been established.
Manifestations of Disease
Clinical Presentation
The characteristic clinical features used to establish the diagnosis of PCOS include (1) ovulatory or menstrual dysfunction, (2) hyperandrogenism, and/or (3) PCOs. The menstrual dysfunction associated with PCOS is generally characterized by infrequent or absent menstrual bleeding and affects 75% to 85% of women affected with the disorder. Menstrual irregularity may begin at menarche or may follow an initial duration of regular periods, and is often hallmarked by oligomenorrhea or amenorrhea. Although menstrual dysfunction is a common presenting symptom of patients with PCOS, between 20% and 30% of women with PCOS will present with eumenorrhea. Patients who present with clinical evidence of hyperandrogenism but give a history of regular menses should have their ovulatory function further evaluated (such as luteal serum progesterone levels). Between 20% and 50% of these women may ultimately be diagnosed with menstrual dysfunction and be considered to be affected by PCOS.
Hyperandrogenism is the most constant and prominent diagnostic component of PCOS. Hyperandrogenism is assessed by clinical features, biochemical indices, or both. The degree to which patients exhibit evidence of hyperandrogenism can vary substantially based on ethnic origin, body weight, and age. Hyperandrogenism is clinically diagnosed by the subjective assessment of hirsutism (≈70%), acne (12% to 40%), and androgenic alopecia (≈10% to 40%). The most commonly used assessment tool to visually score hirsutism is a modification of the method originally reported by Ferriman and Gallwey. Nine body areas, including upper lip, chin, chest, upper and lower back, upper and lower abdomen, and thigh, are visually scored for the density of terminal hairs. A cutoff value of between 6 and 8 has been used to define hirsutism. Biochemically, hyperandrogenemia is most commonly assessed by measurement of the serum testosterone (T) levels, total, and unbound or free. Serum T and sex hormone binding globulin (SHBG) values are used to calculate the free or bioavailable fraction of the free androgen index (T/SHBG × 100). The concentrations of other serum androgens such as androstenedione and dihydroepiandrostenedione sulfate are often high in women with PCOS, but their measurement adds little in diagnostic value in most clinical settings. Other conditions known to mimic the features of PCOS must be excluded. These include nonclassic congenital adrenal hyperplasia, androgen-secreting neoplasms, androgenic–anabolic drug use or abuse, Cushing’s syndrome, the hyperandrogenic-insulin-resistance-acanthosis nigricans syndrome, thyroid dysfunction, and hyperprolactinemia. Although obesity and metabolic syndrome are frequently present in women with PCOS, they are not regarded as intrinsic disturbances of the disorder. Overall between 50% and 70% of women with PCOS have demonstrable insulin resistance.
Imaging Indications and Algorithm
(See later discussion.)
Imaging Technique and Findings
Radiography
No studies demonstrating the usefulness of radiography for patients with PCOS are available in the literature.
Ultrasound
Historic Overview of Imaging for Polycystic Ovaries
Early imaging studies of the ovary were limited by technology (static B-scanner, 3.5 MHz) and utilization of the lower resolution transabdominal approach ( Figure 19-2 ). Those with PCOs or PCOM were noted to have follicles 2 to 6 mm in diameter, but the number was not specifically defined, nor were ovarian stromal characteristics described. US was a tool used to describe the ovarian appearance in women already classified as having PCOS by clinical signs rather than impart information used to establish a diagnosis. With the advent in the 1980s of high-resolution, real-time scanners, more discrete criteria for PCOs and PCOM were established ( Figure 19-3 ). In a seminal article, Adams et al. used two-dimensional (2D) transabdominal ultrasound (TAUS) to define the PCO. The PCO was defined as one in which, in a single plane, at least 10 follicles (2 to 8 mm in diameter) could be seen arranged around a core of echogenic ovarian stroma, or scattered throughout an area of increased stroma ( Figure 19-4 ).



Features of the Polycystic Ovary
ESHRE/ASRM Consensus Definition
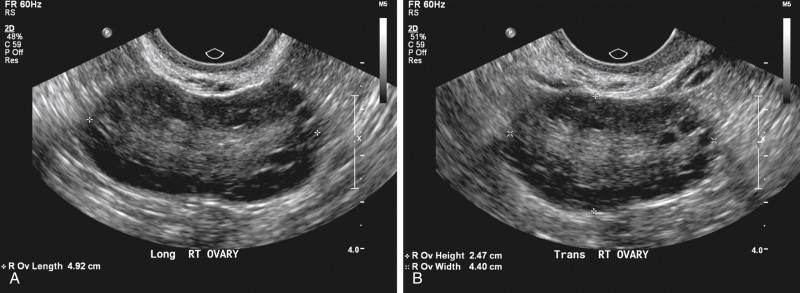
The ESHRE/ASRM consensus workshop definition for the PCO is one that contains 12 or more follicles of 2 to 9 mm in diameter or increased ovarian volume (OV; >10 cm 3 ) ( Figure 19-5 ). To establish the follicular number, each ovary is scanned in a longitudinal plane from the inner to outer margin with the total number of follicles noted. Follicular number is estimated in two planes of the ovary, noting their size and number. The diameter of the follicles is measured as the mean of three diameters (longitudinal, transverse, and anteroposterior). If there is evidence of a dominant follicle (>10 mm) or a corpus luteum, the scan should be repeated in the next cycle. The finding of only one ovary fitting this definition or occurrence of only one of the above criteria (i.e., only follicle number or only volume) is considered sufficient to define PCOM. This definition does not apply to women taking the oral contraceptive pill, as ovarian size is reduced, even though the “polycystic” appearance may persist. The scan should be performed with state-of-the-art equipment, by a transvaginal approach when possible, and in the follicular phase or 3 to 5 days after a progestin-induced bleed or at random within the cycle.

The number of follicles per ovary (FNPO) and size of those follicles accepted to meet the criteria for the PCO were established primarily based on the work of Jonard et al. In a study comparing 214 women with PCOS (oligomenorrhea or amenorrhea, elevated serum luteinizing hormone and/or testosterone, and/or ovarian area [OA] >5.5 cm 2 ) with 112 control subjects, these authors used 2D TVUS to determine total FNPO and to classify follicular size into three different categories (2 to 5, 6 to 9, and 2 to 9 mm). Their study demonstrated that with a threshold set at 12 or more follicles of 2- to 9-mm size, the best compromise was reached with regard to sensitivity (75%) and specificity (99%). Furthermore, in evaluating the subcategories of follicles, this group demonstrated that the mean FNPO was similar between normal ovaries and PCOs in the 6- to 9-mm range, but significantly higher in the PCOs in the 2- to 5-mm and 2- to 9-mm ranges ( Figure 19-6 ). In subsequent work, the size of the 2- to 5-mm follicular pool has been shown to be strongly associated with the severity of the menstrual disorder, and to have a strong negative relationship with the 6- to 9-mm follicular number ( Table 19-2 ).