Fig. 7.1
Pancreatic embryology: the illustration shows progressive embryological development of pancreas as separate dorsal (long arrow) and ventral (short arrow) buds later fusing to form pancreas. The dorsal duct communicated with the bile duct to empty its content into the major papilla
Anatomy and Physiology
The pancreas is a J-shaped elongated organ, located in the retroperitoneum. It is divided anatomically into the head, neck, body, tail, and uncinate process (Fig. 7.2). The head of the pancreas, which comprises 50 % of the pancreatic volume, is located in the C-shaped duodenal loop, lying to the right of the superior mesenteric artery (SMA). The uncinate process can have variable shapes but is usually triangular. The uncinate process lies anterior to the aorta and the inferior vena cava (IVC). The SMA is the boundary between the uncinate process and pancreatic neck. The neck lies anterior to the portal/superior mesenteric vein (SMV) confluence and the SMA. The pancreatic body lies anterior to the lumbar spine, making it prone to direct blunt trauma. There is no clear demarcation of imaging between the pancreatic body and the tail. The proximal tail’s shape and position can vary. The distal tail lies at the splenic hilum.
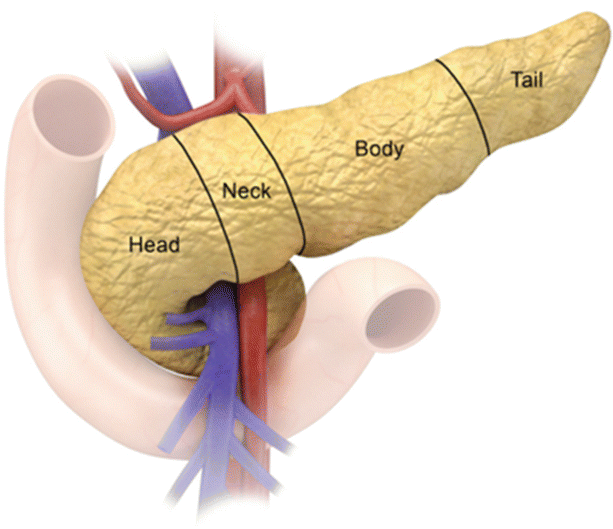

Fig. 7.2
Pancreatic anatomy: the illustration shows the division of the pancreas into head, neck, body, and tail. Note the anatomical relationship of the pancreas to the SMA (Red color) and SMV (Blue Color)
The main pancreatic duct of Wirsung arises from the pancreatic tail and drains the tail, body, neck, and uncinate process. The main duct joins the common bile duct in the pancreatic head before draining into the major papilla of the duodenum. The accessory duct of Santorini mainly drains the head and drains in the minor papilla. The pancreatic ductal anatomy has many variations, which will be discussed later in this chapter.
The pancreatic arterial supply (Fig. 7.3) comes primarily from the celiac artery and the SMA. The gastroduodenal artery, a branch of the common hepatic branch of the celiac axis, gives rise to anterior and posterior divisions of superior pancreaticoduodenal arteries, while anterior and posterior inferior pancreaticoduodenal arteries arise from the SMA. The pancreatic body and tail also derive their blood supply from the splenic or left gastroepiploic arteries. The accompanying superior pancreaticoduodenal veins drain in the portal vein, while the inferior pancreaticoduodenal veins drain into the SMV (Fig. 7.4). Many small venous tributaries also directly drain into the splenic veins.
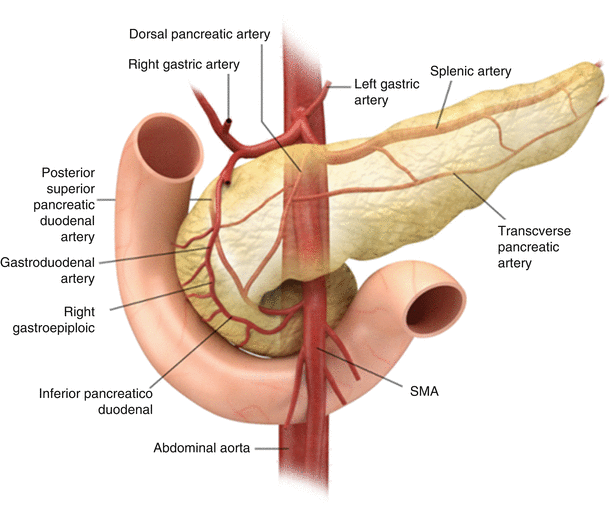
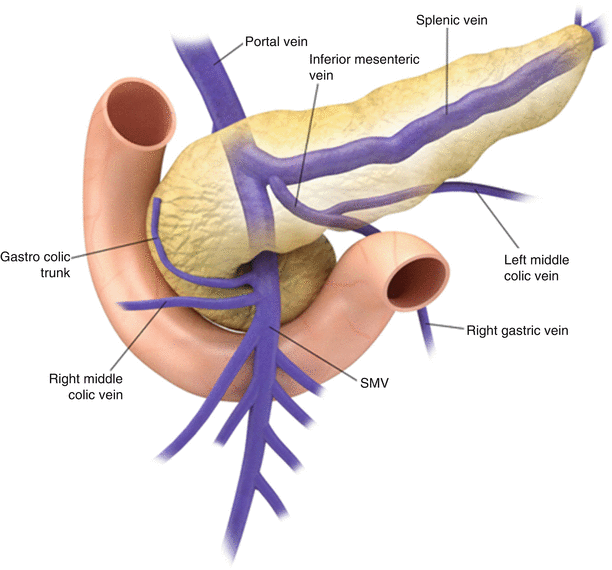

Fig. 7.3
Arterial supply: the illustration shows pancreatic arterial supply

Fig. 7.4
Venous drainage: the illustration shows pancreatic venous drainage
The lymphatic drainage of the pancreas is to the peripancreatic lymph nodes, with nodes along the superior aspect of the pancreatic head receiving its supply from the head, the uncinate process, the neck, and part of the body [7]. The distal pancreas, on the other hand, has lymphatic drainage to the splenic hilar region.
The pancreas has both exocrine and endocrine functions. The exocrine glands consist of numerous acini, secreting contents into intralobular ducts, leading to interlobular or side-branch ducts, finally anastomosing to form the main pancreatic duct. The endocrine glands are comprised of the islets of Langerhans, which are further subdivided into alpha (glucagon), beta (insulin), delta (somatostatin), and pancreatic polypeptide cells, according to their functions.
Imaging Techniques
Computed Tomography (CT)
For pancreatic mass detection, characterization, and preoperative planning (resectability), the dedicated pancreatic mass protocol multi-detector CT (MDCT) is a well-established imaging technique. The technique comprises a triple-phase process: non-contrast, late arterial (10-s delay from peak aortic enhancement), and portal venous (35-s delay) phases [8, 9]. The use of negative contrast (such as water or Volumen) is considered advantageous over positive contrast (such as barium), as bright-appearing barium may mask hemorrhage or calcifications [10]. For acute pancreatitis, an initial assessment could be performed, with the triple-phase exam, although a follow-up exam with a single portal venous phase is usually sufficient [10–12]. Non-contrast imaging helps with delineating calcifications and hemorrhages and can be added in a follow-up exam, for a clinical suspicion of hemorrhagic pancreatitis [12]. Three-dimensional workstations can be used for multiplanar reformatting, maximum or minimum intensity projection, or volume rendering.
Magnetic Resonance Imaging (MRI)
The pancreatic MRI protocol primarily comprises breath-hold three-dimensional T1-weighted gradient echo imaging, T2-weighted imaging, and dynamic fat-suppressed post-contrast T1-weighted imaging sequences. Thin- and thick-slab heavily T2-weighted MR cholangiopancreatography (MRCP) sequences can help in delineating ductal anatomy and better characterize cystic lesions and their relations to ducts [13, 14]. Diffusion-weighted images are routinely performed to assist in characterizing focal or diffuse pancreatic disorders [15]. The inherent bright T1 signal of the pancreas makes T1-weighted imaging useful for detecting pancreatic adenocarcinomas, which are usually hypointense. Early changes of pancreatic fibrosis in the setting of chronic pancreatitis are also well seen on T1-weighted images and are hypointense to the pancreatic parenchyma [16, 17].
Positron-Emission Tomography (PET)-CT
PET-CT using fluorine-18 2-deoxy-2-fluoro-D-glucose (FDG) can be useful in pancreatic cancer staging and restaging. FDG PET-CT has also been shown to be useful in assessing recurrent pancreatic cancer when the CT findings are equivocal, specifically in the setting of rising cancer antigen 19-9 (CA 19-9) [18–20]. Pancreatic cancer may or may not show FDG avidity.
Classification of Pancreatic Disorders
The pancreatic disorders can be broadly classified into congenital, inflammatory, traumatic, and neoplastic etiologies (Algorithm 7.1).
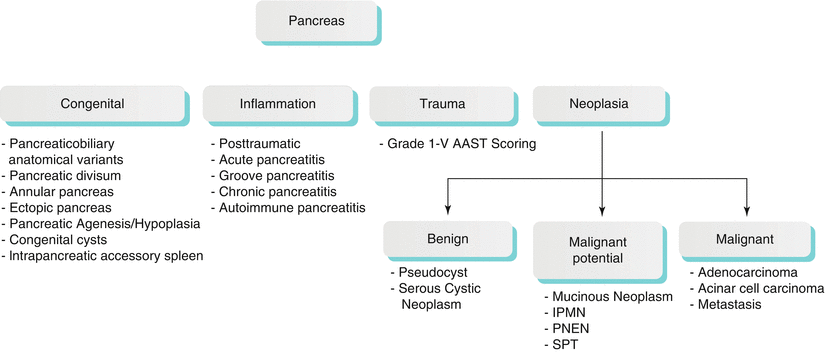

Algorithm 7.1 Pathological Classification of Common Pancreatic Disorders
Congenital Pancreatic Disorders
Pancreaticobiliary Anatomical Ductal Variants
Anomalous pancreaticobiliary union (APBU) is diagnosed when the pancreatic and biliary ducts join outside of the duodenum. This malunion of the pancreatic and bile duct junctions allows free reflux of pancreatic secretions into the biliary system. APBU is clinically significant due to increased risk for pancreatitis and choledochal cysts (which increase the risk of underlying neoplasms including cholangiocarcinoma); these patients are usually offered surgical treatment [21]. With better techniques and thin-section imaging, this anomaly is being increasingly seen as an incidental finding. MRI, MRCP, and T2-weighted fast spin echo sequences are the best sequences to evaluate ductal anomalies on MRI. On cross-sectional imaging, attempts should be made to carefully evaluate anomalies in patients with recurrent pancreatitis. Many other pancreatic-biliary ductal anomalies exist, which are beyond the scope of this chapter.
Pancreatic Divisum
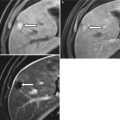
In pancreatic divisum, the dorsal pancreatic duct fails to connect to the ventral counterpart during embryogenesis and instead continues as the duct of Santorini, which is of a smaller caliber than the duct of Wirsung (Figs. 7.5 and 7.6). A minority of such patients can develop recurrent pancreatitis, and this subset of the patient population can be potentially treated endoscopically [22, 23]. In cross-sectional imaging, sometimes the distinct unfused dorsal and ventral pancreatic moieties can be seen as separate structures separated by a fat cleft.
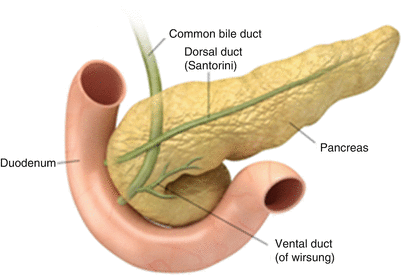
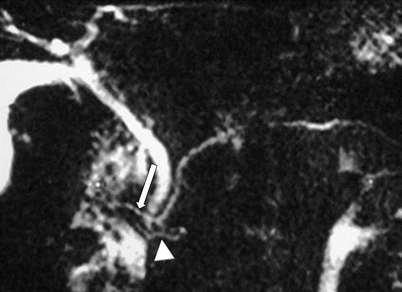

Fig. 7.5
Pancreatic divisum: the illustration shows continuation of the main pancreatic duct as the duct of Santorini draining into the minor papilla. Additionally, the duct of Wirsung is not communicating with the main duct

Fig. 7.6
Pancreatic divisum: MR cholangiopancreaticogram sequence demonstrates two separate, communicating dorsal (arrow) and ventral (arrowhead) ductal systems, compatible with a pancreatic divisum
Annular Pancreas
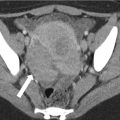
Annular pancreas is a fusion anomaly of the dorsal and ventral pancreatic buds that causes the pancreatic head to encircle the second part of the duodenum (Fig. 7.7). Annular pancreas is most often diagnosed prenatally or in infancy secondary to symptomatic duodenal obstruction [24]. Sometimes, symptoms do not present until adulthood and may manifest as duodenal obstruction or recurrent pancreatitis. The diagnosis can also be made incidentally on imaging. On fluoroscopic upper gastrointestinal series or small bowel follow-through, it manifests as a smooth-appearing periampullary duodenal constriction. On cross-sectional imaging, this finding can be subtle and appears as encircling pancreatic parenchyma around the duodenum, and careful evaluation of the periampullary region is necessary if there is a strong clinical suspicion (Fig. 7.8). Additionally, the peripancreatic region and the pancreas should be carefully evaluated to not overlook changes suggestive of subtle acute or chronic pancreatitis (peripancreatic stranding or atrophy of the pancreas) or biliary obstruction [23].
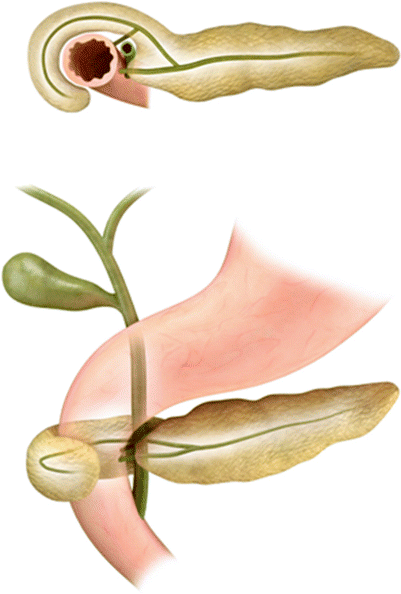
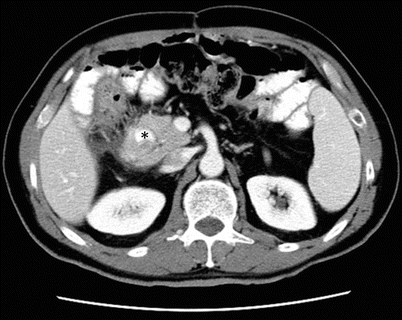

Fig. 7.7
Annular pancreas: the illustration shows abnormal fusion of dorsal and ventral pancreatic buds (Refer to Fig. 7.1) encircling the second part of the duodenum

Fig. 7.8
Annular pancreas: axial contrast-enhanced CT image shows pancreatic tissue encircling the second part of the duodenum (asterisk) which is filled with oral contrast
Ectopic Pancreas
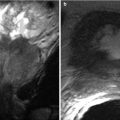
Also known as pancreatic rests, ectopic pancreatic tissue has been found in the stomach (classically the antrum), duodenum, and small bowel (including Meckel’s diverticulum). It is rarely symptomatic and is very rarely seen on cross-sectional imaging as a discrete finding (Fig. 7.9).
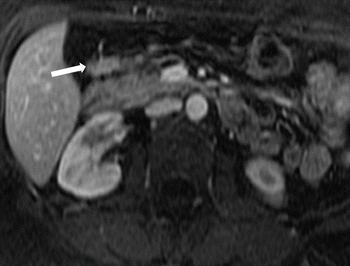

Fig. 7.9
Ectopic pancreas: post-contrast T1W MR image demonstrates pancreatic tissue in an ectopic location along the posterior aspect of the gastric antrum (arrow)
Pancreatic Agenesis/Hypoplasia
Pancreatic hypoplasia (partial agenesis) is usually seen as the absence of dorsal pancreas and can present as an incidental finding or as neonatal or early-onset diabetes mellitus. In clinically suspected cases of pancreatic hypoplasia, MRI is the best modality for evaluation as it has better contrast resolution and is free of radiation hazards.
Congenital Cysts
Congenital cysts localized to the pancreas are very rare and usually are associated with cystic changes in other organs [25]. When imaging adults, these lesions can be difficult to distinguish from pseudocysts or mucinous neoplasms in the absence of remote comparison examinations or a history of chronic pancreatitis or other clinical syndromes such as polycystic kidney disease, von Hippel-Lindau syndrome, and tuberous sclerosis that can lead to pancreatic cysts.
Inflammatory Disorders of the Pancreas
Acute Pancreatitis
There are numerous etiological factors of acute pancreatitis (Table 7.1); it may result from alcoholism, cholelithiasis, and iatrogenically from endoscopic retrograde cholangiopancreatography (ERCP). Altered pancreatic ductal permeability or direct toxic effects from inciting agents (such as alcohol and drugs) cause leakage of lipase and amylase, which exposes the pancreatic and peripancreatic tissues to injury. The pathologic spectrum of acute pancreatitis ranges from microscopic interstitial form to fat necrosis, ultimately leading to systemic inflammatory response syndrome that might cause death.
Table 7.1
Etiological factors for acute pancreatitis
Type | Factor |
|---|---|
Common | Gallstones (35–55 %) |
Alcohol (25–40 %) | |
Uncommon | Iatrogenic (ERCP) (3–5 %) |
Rare | Trauma/surgery |
Medications | |
Viral infection | |
Metabolic disorders | |
Hereditary pancreatitis | |
Congenital anomalies induced | |
Obstructive pancreatitis (mass or calculus) | |
Collagen vascular diseases |
The incidence of acute pancreatitis has been on the rise over the last several years. There were 51,895 hospital admissions related to acute pancreatitis in 2006, as compared to 36,510 cases in 1998, which can be primarily attributed to an increase in the incidence of gallstones due to increasing obesity [26].
Acute Pancreatitis Classification Systems
The progression of acute pancreatitis to severe pancreatitis can be seen in 10–20 % of cases, and 2–10 % of cases of acute pancreatitis can present as a lethal episode [27, 28]. Early diagnosis of pancreatitis and assessment (clinically and radiologically) of severity plays a significant role in deciding the management course and thereby reducing morbidity and mortality related to acute pancreatitis. In this section, we will describe the commonly used clinical and imaging scoring systems that have been developed and revised over the last few decades.
Clinical Scoring Systems
There are numerous clinical and laboratory markers that determine acute pancreatitis severity [27–30]. Independent assessment of these parameters is not practical, and therefore many clinical parameters are proposed. Ranson’s and APACHE (acute physiology and chronic health examination) II scoring systems are commonly used [31, 32].
Ranson’s criteria take 11 parameters (5 parameters to be evaluated at the time of admission and 6 parameters after 48 h) into consideration, as noted in Table 7.2. The mortality directly correlates with an increasing Ranson’s score, with a 40 % risk of death if the score is greater than or equal to 6 [27, 31]. APACHE II scoring is commonly used to assess the severity of acute pancreatitis in patients. It uses 12 clinical and laboratory parameters, with an increasing score directly proportional to mortality [27, 32].
Table 7.2
Ranson’s criteria for acute pancreatitis
At admission | After 48 h of admission |
|---|---|
Age >55 years | Hematocrit level fall >10 % |
White blood cell count >16,000/uL | Increase in blood urea nitrogen >5 mg/dl |
Serum glucose >200 mg/dl | Serum calcium <8 mg/dl |
Lactate dehydrogenase >350 IU/L | pO2 <60 mmHg |
Aspartate aminotransferase >250 IU/L | Base deficit >4 mEq/L |
Fluid sequestration >6 L | |
Total score: up to 5 | Total score: up to 6 |
CT Severity Index
The CT severity index was described by Balthazar et al. [33], based on which patients can be divided into low-, medium-, or high-severity groups (Table 7.3). Although this was a major advance in acute pancreatitis management, there are many limitations of this index, including lack of correlation with extrapancreatic parenchymal or vascular complications and subsequent development of organ failure [27, 34–36].
Table 7.3
Balthazar’s CT severity index for acute pancreatitis
CT findings | Score |
|---|---|
Extent of pancreatic inflammation | |
Normal pancreas | 0 |
Pancreatic enlargement, focal or diffuse | 1 |
Grade B with peripancreatic inflammation | 2 |
Grade C with single peripancreatic fluid collection | 3 |
Grade C with two or more peripancreatic fluid collection | 4 |
Pancreatic necrosis | |
0 % | 0 |
≤30 % | 2 |
>30–50 % | 4 |
>50 % | 6 |
Revised Atlanta Classification System
Early Phase Versus Late Phase
From an imaging perspective, the revised Atlanta classification system is the most commonly adapted one. This classification system clearly distinguishes the early pancreatitis phase (first week), in which management is driven by clinical parameters, from a late phase (after the first week), in which the treatment strategy is based on imaging criteria in addition to clinical parameters [37–40]. Clinically, the determination of severity can be based on a scoring system such as the Ranson’s or APACHE II systems.
An early phase is typically not readily identified in imaging in many cases. CT images can be completely normal in 15–30 % of cases of uncomplicated pancreatitis, and this number could be higher if the CT examination is performed in the first 24 h [27]. If positive, the common CT findings in the early phase include pancreatic enlargement, pancreatic margin blurring, or peripancreatic fat stranding (Fig. 7.10). Chronic pancreatitis changes might coexist. MR imaging findings would include loss of inherent bright T1 signal, pancreatic enlargement, peripancreatic blurring, fat stranding, or an increased signal on T2-weighted images or low b-value diffusion-weighted images.
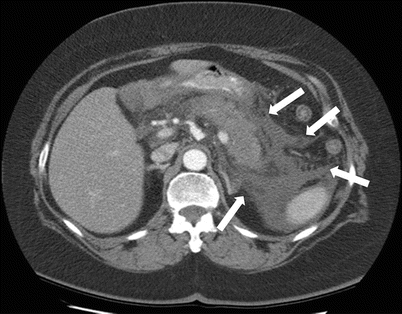

Fig. 7.10
Acute pancreatitis: axial contrast-enhanced CT image demonstrates pancreatic margin blurring, loss of lobulations, and associated pancreatic and peripancreatic fatty necrosis (arrows)
The late phase typically begins after the first week and can exist for a period from a few weeks to several months. Common complications include necrosis (simple or hemorrhagic), superimposed infection, and multiorgan failure. The imaging in this phase provides crucial information to guide the management of treatment. The mortality can increase to 30 % for cases complicated with superimposed infection [27]. CT is the mainstay modality in this setting, particularly in the setting of clinical progression and for image-guided procedures. When using CT, one should particularly assess for necrosis, infection, extent of fat necrosis, developing pseudocysts, other organ involvement, biliary complications, or vascular complications [38, 39]. The role of MRI is mainly as a problem-solving modality, with indications including but not limited to identifying potential causes of pancreatitis such as gallstones/choledocholithiasis, occasionally characterizing fluid collections, or evaluating for pancreatic ductal leak.
Morphological Classification
The morphological classification of acute pancreatitis per the revised Atlanta classification is shown in Algorithm 7.2. Acute pancreatitis is now subdivided into acute interstitial edematous pancreatitis (AIEP) and acute necrotizing pancreatitis (ANP). AIEP is seen as focal or diffuse homogeneous (sometimes heterogeneous) enlargement on CT, which can sometimes be associated with mild peripancreatic stranding and small fluid collections.
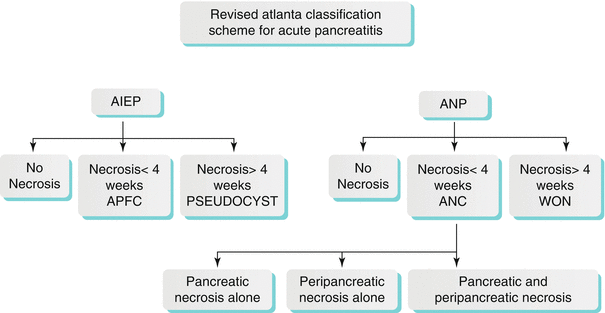

Algorithm 7.2 Revised atlanta classification scheme for acute pancreatitis
ANP is subdivided into pancreatic parenchymal necrosis with peripancreatic necrosis (75–80 %), peripancreatic necrosis alone (20 %), and pancreatic parenchymal necrosis alone (5 %) [38–40]. The pancreatic parenchymal necrosis on CT appears as one or more areas of non-enhancement (Fig. 7.11). Physicians often underestimate the extent of the pancreatic necrosis in early phases when reviewing CT images; therefore, in the right clinical scenario, CT should be repeated a few days after the first scans. On the other hand, physicians may overestimate the findings in early-phase CT scans, as edema can sometimes produce areas of decreased enhancement; therefore, follow-up study may be helpful. Also, careful attention should be made for hemorrhagic transformation or superimposed infection. Peripancreatic necrosis is commonly seen in the retroperitoneum and in the lesser sac. The peripancreatic necrosis carries a better prognosis than pancreatic parenchymal necrosis but a less favorable one than AIEP.
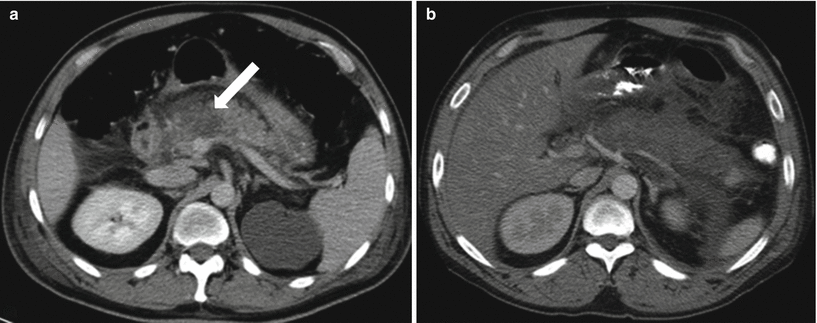

Fig. 7.11
Acute pancreatitis: contrast-enhanced axial CT image (a) shows geographic area of parenchymal non-enhancement (arrow) in the region of the pancreatic neck and part of the body, compatible with necrosis in the setting of acute pancreatitis (note peripancreatic stranding and fluid). Image (b) shows a greater extent of pancreatic necrosis in a different patient, involving most of the visualized pancreas
Pancreatic and Peripancreatic Fluid Collections
The revised Atlanta classification describes five distinct types of fluid collections [37–40]. In the acute setting, the collections are either acute peripancreatic fluid collections (APFCs) or acute necrotic collections (ANCs); both are typically less than 4 weeks old. When complicated, AIEP can present with APFCs, which, over time (greater than 4 weeks), can lead to pseudocyst formation. ANP, in its all three subtypes, can be complicated by ANCs, which over time could persist as walled-off necrosis (WON). Superimposed infection can complicate all of these collections. WON is typically formed at or over 4 weeks from the maturation of ANCs. Similar to the need for ANCs, it is important to differentiate collections containing necrotic material from collections containing simple fluid on imaging, as the non-liquefied component of the collection needs to be removed (often surgically), while pseudocysts are almost always simply treated by drainage.
APFCs on contrast-enhanced CT appear as a simple fluid collection confined by peripancreatic fascial planes, do not show necrotic components or debris, and have no defined wall. Therefore, such collections within the pancreas should never be diagnosed as APFCs. Since these collections do not have a wall, they tend to resolve with time and most importantly can get superinfected following intervention; thus imaging-guided drainage is typically not recommended [38–40]. ANCs, on the other hand, are believed to result from fat saponification, secondary to the release of activated pancreatic enzymes. Therefore, ANCs typically appear heterogeneous with layering debris. In equivocal cases, an ultrasound or MRI scan can help identify necrotic changes and may be able to find the foci of the fat in the ANCs.
Pseudocyst is defined as a fluid collection enclosed by an encapsulated wall of granulation tissue, with little or no necrosis. It takes more than 4 weeks to form a pseudocyst from APFC. On contrast-enhanced CT, it appears as a simple fluid collection with a thin, well-defined wall (Fig. 7.12). MRI might be needed to confirm the absence of pancreatic necrosis and to document communication of the collection with the main pancreatic duct. The presence of communication of the pseudocyst to the ductal system would suggest that it might not be able to be resolved with conservative treatment management and that drainage or surgical management might be required in selected cases [38, 39]. Pseudocysts do not commonly occur in the setting of a single episode of acute pancreatitis; they are usually seen in patients who have chronic pancreatitis with superimposed recurrent episodes of acute pancreatitis.
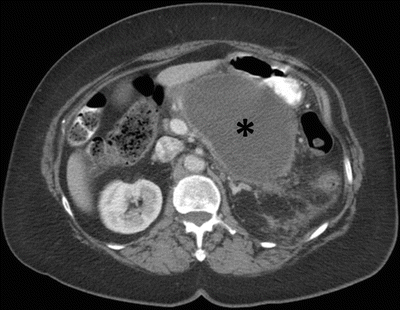

Fig. 7.12
Acute pancreatitis: contrast-enhanced axial CT image in a patient with recent history of acute pancreatitis (>4 weeks) shows a fluid collection (asterisk) in the peripancreatic region with perceptible wall, compatible with a pseudocyst
Acute Pancreatitis Complications
Superimposed infection is a common complication from both AIEP and ANP. Infection can complicate 40–70 % of cases of severe pancreatitis, and typical onset is 10–14 days after incidence of severe pancreatitis [27, 38]. Infection further potentiates the already high mortality of severe pancreatitis to up to 80 % [27]. The most specific imaging finding on CT scans is the presence of gas (Figs. 7.13 and 7.14). Imaging also helps in management planning that includes percutaneous drainage versus surgical treatment.
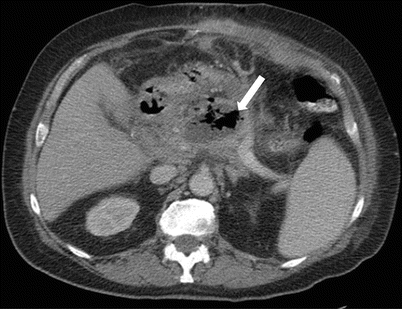
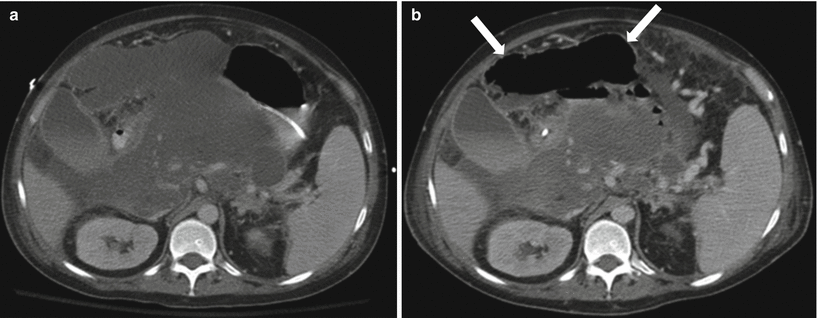

Fig. 7.13
Acute pancreatitis: contrast-enhanced axial CT image in a patient with history of acute pancreatitis shows a complex fluid collection in the peripancreatic region containing gas (arrow), which would be concerning for superimposed infection

Fig. 7.14
Acute pancreatitis: contrast-enhanced axial CT image (a) in a patient with history of acute pancreatitis shows large peripancreatic fluid collections in the peripancreatic region. Follow-up image (b) a few days later shows interval development of large amount of gas (arrows) in the largest collection, which would be concerning for superimposed infection
Hemorrhage complicates acute pancreatitis in 1.2–14.5 % of cases and has a high mortality rate [27, 38, 39]. In early phases, hemorrhage is usually secondary to vascular wall damage by pancreatic enzymes. In later phases, the mechanism is usually erosion by pseudocyst, WON, or abscess; capillary bleeding from cyst/WON wall; or, rarely, erosion into the bowel wall [38, 39]. On CT, hemorrhage is readily identifiable by high-density material, often layering in the cyst or necrotic cavity (Fig. 7.15). The pseudoaneurysm formation is usually a chronic complication and can involve the splenic, pancreaticoduodenal, and gastroduodenal arteries [41, 42]. It is very important to identify pseudoaneurysms on cross-sectional imaging, as untreated pseudoaneurysms have high mortality [42] (Figs. 7.15 and 7.16).
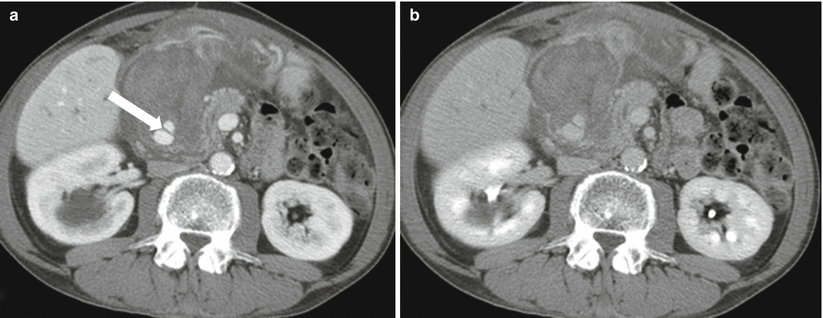
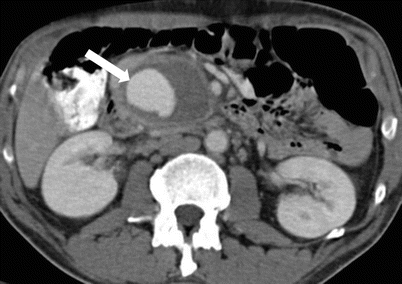

Fig. 7.15
Acute pancreatitis: contrast-enhanced axial CT images (a, b) in a patient with recent history of acute pancreatitis shows focus with enhancement similar to the aorta (arrow) compatible with a pseudoaneurysm (of one of the pancreaticoduodenal branches). In this case, there is extensive acute hemorrhage surrounding this pseudoaneurysm as noted by dense, complex swirling pattern. Untreated pseudoaneurysms can have high mortality and need to be managed aggressively

Fig. 7.16
Acute pancreatitis: contrast-enhanced axial CT image in a patient with recent history of acute pancreatitis shows a pseudoaneurysm from one of the pancreaticoduodenal branches (arrow). In this case, there is contained hematoma surrounding the pseudoaneurysm. Untreated pseudoaneurysms can have high mortality and need to be managed aggressively
Pancreatitis can also result in venous thrombosis and is seen in approximately 13–24 % cases and most commonly involves the splenic veins and if chronic can lead to portal hypertension [27, 42] (Fig. 7.17).
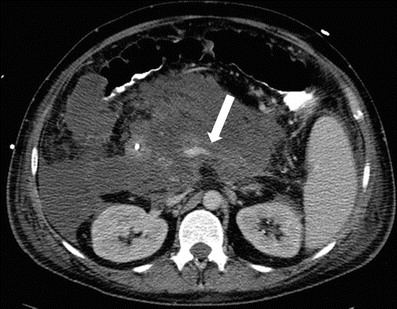

Fig. 7.17
Acute pancreatitis: contrast-enhanced axial CT image in a patient with acute pancreatitis shows a filling defect (arrow) in the splenic vein extending into the portal venous confluence, compatible with venous thrombosis as a complication from acute pancreatitis. Note extensive peripancreatic stranding and fluid collections
Chronic Pancreatitis
Chronic pancreatitis is defined as progressive, irreversible, and permanent damage of the pancreatic glands that leads to the impairment of both endocrine and exocrine functions of the gland. The various etiological factors (the most common factor is alcohol) ultimately lead to the loss of parenchymal cells, repetitive sustained chronic inflammation, and fibrosis [43–45]. Recurrent epigastric pain, steatorrhea, and diabetes mellitus are common presentations.
The sensitivity of CT varies from 75 to 90 % for diagnosing chronic pancreatitis [46, 47]. Pancreatic ductal dilatation (more than 5 mm in the pancreatic head and 2 mm in the pancreatic body and tail) in the absence of an obvious mass is seen on CT images in early chronic pancreatitis. The dilatation is sometimes seen in combination with mild pancreatic atrophy. Coronal reconstruction adds to the confidence for diagnosing ductal dilatation. More advanced CT features include irregularity of the dilated duct, pseudocyst formation, parenchymal heterogeneity, irregular contour of the head and body, focal parenchymal necrosis, pancreatic calcifications, ductal stricture, and contiguous organ invasion (Fig. 7.18).
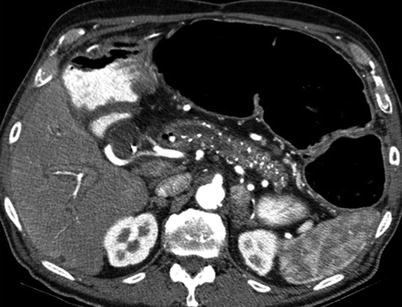

Fig. 7.18
Chronic pancreatitis: contrast-enhanced axial CT image demonstrates diffuse calcifications in the pancreatic parenchyma, with irregular pancreatic ductal dilatation, compatible with chronic pancreatitis
MR imaging with MRCP is more sensitive and specific than CT for diagnosing chronic pancreatitis, as it provides better tissue contrast resolution [48, 49]. On T1-weighted images, the pancreatic parenchyma loses the inherent bright T1 signal. Foci of signal voids on T1- and T2-weighted sequences suggest calcification. MRCP is the best MRI sequence(s) to evaluate the pancreatic ductal system [50, 51]. Secretin-induced pancreatic MRCP is performed in selected cases to assess pancreatic ductal function. It has more than a 90 % correlation with ERCP findings [50]. Post-secretin MRCP can add significant value to the MRI assessment of pancreatic physiology, which can be evaluated by a qualitative assessment of ductal flow into the duodenum, ductal caliber changes, and acinar filling.
Chronic pancreatitis often can produce an appearance of focal mass [52, 53] (Fig. 7.19). On imaging, the mass-forming pancreatitis may appear higher or lower in attenuation compared to the normal pancreatic parenchyma and may show early arterial enhancement, depending on the ratio of fibrous or cellular components [53]. On CT images, focal pancreatic enlargement associated with calcifications and ductal dilatation is highly suggestive of chronic pancreatitis. However, quite often it is difficult to exclude an underlying mass, as pancreatic cancer can develop within chronic pancreatitis. In equivocal cases, FDG PET-CT might sometimes be helpful, as pancreatic carcinoma may be metabolically active and chronic pancreatitis may not be [54, 55].
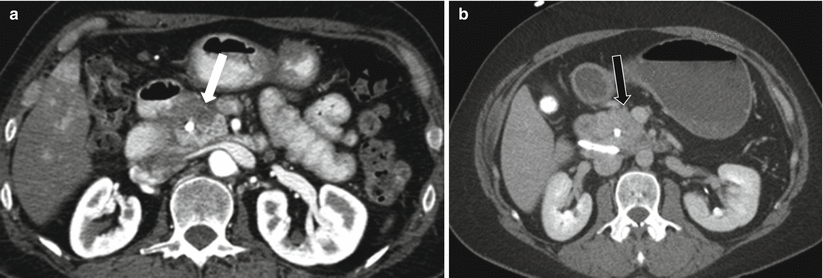

Fig. 7.19
Chronic pancreatitis: contrast-enhanced axial CT images in early arterial (a) and 5-min-delayed phases (b) on a patient demonstrate mass-like abnormality in the pancreatic head (white arrow), causing biliary obstruction (biliary stent in place). Note the enhancement on delayed phase (black arrow), suggesting presence of predominantly fibrous tissue. Multiple biopsy attempts failed to reveal malignancy
Autoimmune Pancreatitis (AIP)
AIP is a distinct type of chronic pancreatitis (2–11 %), with infiltration of immunoglobulin G4 (IgG4)-positive plasma cells, leading to pancreatic fibrosis and dysfunction [56–58]. It is more commonly seen in men (2:1) and usually manifests in middle age. Patients usually present with jaundice without pain or with some discomfort.
In 2003, Kamisawa et al. suggested that AIP might fall within the spectrum of IgG4-related sclerosing disease [59]. The extrapancreatic findings in this spectrum might involve the biliary system, kidneys, retroperitoneum, mesentery, thyroid, lacrimal gland, orbits, salivary glands, lymph nodes, lungs, gastrointestinal tract, and vascular system. With better awareness of this spectrum, increasing imaging use, better imaging techniques, and increasing incidence of imaging findings are being noted in various organs affected by IgG4-related sclerosing disease. Imaging is also useful in assessing responses to steroids, the most common therapy for IgG4-related sclerosing disease.
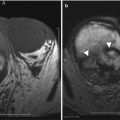
The AIP most commonly involves the entire pancreatic gland, which appears sausage-shaped due to pancreatic enlargement, sharp borders, loss of lobulations, and pancreatic clefts (Fig. 7.20). The pancreatic duct is not dilated in these patients. The focal type of AIP can mimic malignancy on imaging and can be very difficult to differentiate.
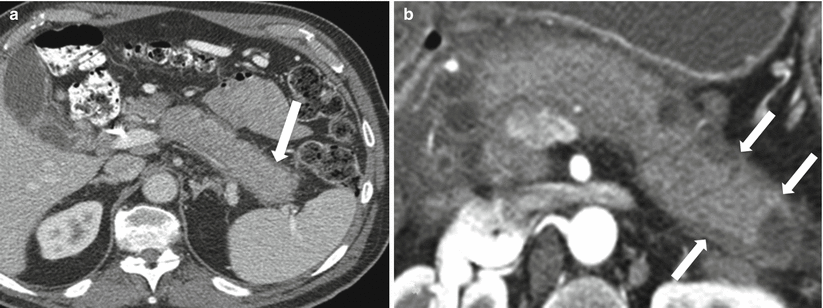

Fig. 7.20
Autoimmune pancreatitis: contrast-enhanced axial CT images in different patients (a, b) demonstrate diffuse enlargement of the pancreas, which appears sausage-shaped and demonstrates thin peripancreatic capsule (arrows)
On CT, the affected region appears hypodense due to edema, demonstrates less enhancement in early phases as compared to avid pancreatic enhancement otherwise, and can show delayed enhancement due to fibrosis (Figs. 7.20 and 7.21). The pancreas appears sausage-shaped with a thin surrounding rim or halo [60, 61]. As opposed to a common presentation of chronic pancreatitis with an irregular dilated duct, the duct in AIP tends to be narrow and irregular. It can be very difficult to identify and characterize the pancreatic duct on CT imaging.
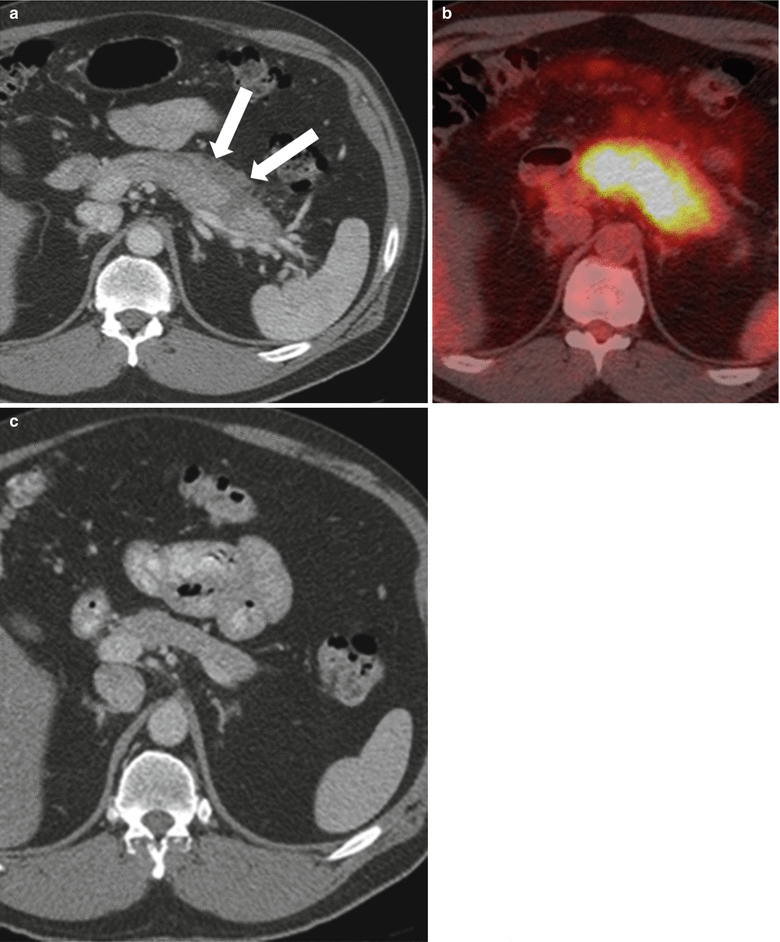

Fig. 7.21
Autoimmune pancreatitis: contrast-enhanced axial CT image (a) on a patient demonstrates diffuse enlargement and FDG avidity (b) of the pancreas. Note heterogeneous enhancement of the pancreas and thick peripancreatic capsule (arrows) on image a. The diagnosis of autoimmune pancreatitis was considered; the patient did receive steroids and follow-up CT exam (c) showed improvement and finally resolution of pancreatic changes
MR imaging shows a T1-hypointense signal and a T2-hyperintense signal in affected pancreas. As with CT, MRI would also show early hypoenhancement of the affected region due to edema, followed by delayed hyperenhancement due to fibrosis. MRCP can add value when evaluating ductal morphology [61].
It is worth remembering the presence of a multisystem disease process within the spectrum of IgG4-related sclerosing disease. Therefore, particular attention should be made to the hepatobiliary system (sclerosing cholangitis, gallbladder wall thickening), kidneys (renal cortical masses, pericortical masses, renal pelvis involvement), retroperitoneum (fibrosis), mesentery (sclerosing mesenteritis), gastrointestinal tract (inflammatory bowel disease), lymph node (enlargement), and vascular system (vasculitis), particularly because these structures are within the same field of view of pancreatic imaging [62–64].
Groove Pancreatitis
Groove pancreatitis is a rare entity, presenting in acute or chronic form, characterized as focal inflammation or scarring between the pancreatic head, duodenal wall, and common bile duct [65]. In a chronic setting, there is an increased risk of common bile duct or duodenal strictures [66]. Additionally, this can be very difficult to differentiate from a pancreatic head neoplasm. On CT images, the appearance of groove pancreatitis is that of focal acute or chronic pancreatitis in this classic location (Fig. 7.22).
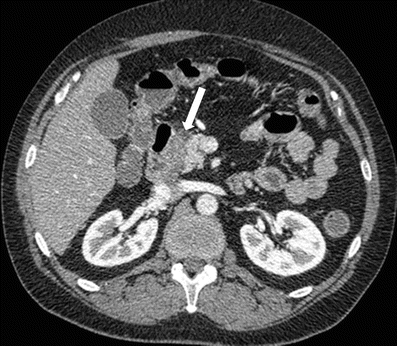

Fig. 7.22
Groove pancreatitis: contrast-enhanced axial CT image shows hypoattenuating lesion in pancreaticoduodenal groove (arrow), with some mass effect on the pancreatic head and duodenum
Pancreatic Trauma
Blunt trauma to the pancreas can commonly occur from a motor vehicle accident. It is, however, the least commonly injured abdominal organ (less than 2 % of injuries involving the pancreas) and often coexists with other organ injuries [67, 68]. Coexisting injuries to other organs, hemorrhage, infection, and multiorgan failure are leading causes of death among patients with pancreatic injury. The blunt trauma results in crushing of the retroperitoneal pancreas against the spine; therefore, pancreatic injury is often associated with duodenal injury [67].
CT is the mainstay modality due to its easy and fast availability. As in acute pancreatitis, the pancreatic traumatic injury can be invisible in early phases on CT images. In an appropriate clinical setting, repeat CT can be performed. The important CT findings would include pancreatic contusions (focal or diffuse hypoenhancement on a contrast-enhanced exam), peripancreatic stranding, pancreatic laceration or disruption (linear hypoenhancing areas within the pancreas) (Fig. 7.23), pancreatic ductal discontinuity, hematoma (heterogeneous or hyperdense areas, best characterized on the non-contrast phase of a CT exam), pseudoaneurysm formation, and splenic venous thrombosis [67].
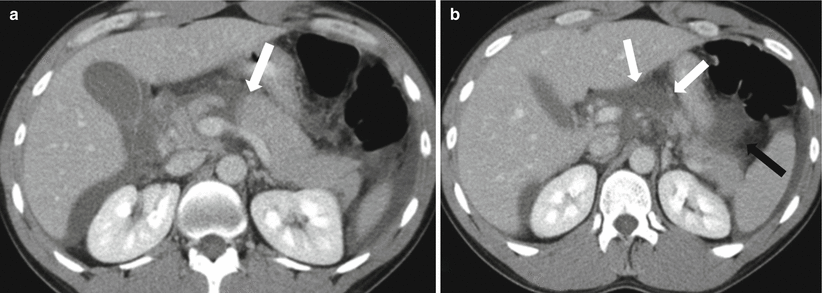

Fig. 7.23
Pancreatic transection: contrast-enhanced axial CT images (a, b) show linear low-density area (white arrow) through the pancreatic neck area, separating the body from the head of the pancreas. Note extensive peripancreatic fluid (black arrows)
The most adapted system for pancreatic injury grading is the one published by the American Association for the Surgery of Trauma (AAST) [67, 68] (Table 7.4).
Table 7.4
AAST pancreatic trauma classification
Grade | Type of injury | Description of injury |
|---|---|---|
I | Hematoma | Minor contusion without ductal injury |
Laceration | Superficial laceration without ductal injury | |
II | Hematoma | Major contusion without ductal injury |
Laceration | Major laceration without ductal injury or tissue loss | |
III | Laceration | Distal transection or parenchymal injury with ductal injury |
IV | Laceration | Proximal transection or parenchymal injury involving ampulla |
V | Disruption | Massive disruption of pancreatic head |
MRI can be very helpful in subtle cases. Pancreatic edema can be best recognized on a T2-weighted sequence. MRI can, in particular, help to identify subtle ductal injuries on MRCP sequences, without—and in some cases with—the secretin challenge test.
Pancreatic Deposition Disorders
The pancreas can be fatty-replaced on cross-sectional imaging in elderly patients and may be associated with pancreatic atrophy, in which cases there is insinuation of fat in between the pancreatic parenchyma. Pathological fatty deposition can be seen in patients with obesity, steroid use, malnutrition, alcoholic hepatitis, and certain hereditary syndromes related to primary exocrine insufficiency, such as Shwachman-Diamond syndrome or cystic fibrosis (Fig. 7.24) [69]. When diffuse, it is easy to recognize on cross-sectional imaging by fat density on the non-contrast phase of a CT exam and by loss of signal on fat-saturated T1 images on an MRI exam. When focal, this can mimic malignancy, and secondary signs described in the pancreatic carcinoma section of this book are helpful in differentiating the two pathologies at extremes of the spectrum. Chemical-shift MRI sequences can be particularly helpful in this setting [69, 70].
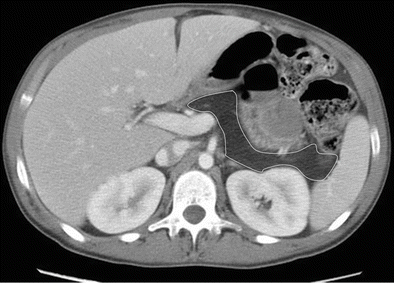

Fig. 7.24
Fatty replacement of the pancreas: contrast-enhanced axial CT image shows complete replacement of the pancreas by fatty tissue in this patient with history of cystic fibrosis. The expected location of the pancreas is delineated with white line drawing
Primary hemochromatosis predominantly involves the liver, pancreas, and myocardium, but not the spleen [69]. Pancreatic dysfunction does not directly correlate with the amount of deposition. CT has classically demonstrated hyperattenuation of pancreatic parenchyma on non-contrast exams, although it is far less sensitive than on MR images. On MRI, the pancreas affected by hemochromatosis would lose signal on T1-weighted gradient echo images (with sequentially increasing echo time used for hepatic iron load quantification) or on T2-weighted images [69, 71].
Pancreatic Transplantation
Pancreatic transplantation alone or in combination with kidney transplantation is a very complex procedure, with the most common indication being type I diabetes mellitus. The pancreas is usually transplanted in the pelvis, with an arterial supply from and venous drainage to external iliac vessels. With improvement in the technique, improving posttransplant management with immunosuppression, and better imaging techniques and experience, the 1-year pancreatic graft survival has been improving over the last decade [69, 72].
The role of imaging in pancreatic transplant evaluation is typically in identifying postoperative complications or transplant rejection. Ultrasound with Doppler is a good initial imaging modality in evaluation, particularly in the immediate postoperative period during which vascular compromise and rejection are primary concerns, and it can be performed at bedside [73–75]. Parenchymal grayscale evaluation is not optimal with ultrasound, particularly in the immediate postoperative setting (primarily due to overlying bowel gas and difficult patient positioning which limits ultrasound evaluation). On Doppler evaluation along with spectral waveform analysis, vascular compromise such as arterial anastomotic stenosis, arterial or venous thrombosis, and formation of pseudoaneurysms can be identified. Cross-sectional imaging is helpful in cases of suspected infection or inflammation or in cases in which the ultrasound fails to demonstrate the etiology for clinical suspicion of graft dysfunction [74, 75]. In postoperative imaging, CT imaging can show postsurgical fluid collections, transplant inflammation/necrosis, arterial stenosis, venous thrombosis, and superimposed infections as complications.
Pancreatic Neoplasms
Pancreatic neoplasms are primarily classified into cystic and solid pancreatic neoplasms (Algorithm 7.1).
Cystic Pancreatic Neoplasms
Although cystic tumors constitute only 10 % of pancreatic neoplasms, with the increasing use of cross-sectional imaging of the abdomen and better techniques, the cystic lesions in the pancreas have become a common incidental finding, frequently representing neoplasms [76–78]. Accurate recognition and characterization is important since, unlike primary adenocarcinoma, cystic neoplasms of the pancreas are highly curable. The majority of these lesions represent pseudocysts, serous and mucinous cystic neoplasms, and IPMNs (Algorithm 7.3). Pancreatic islet-cell tumors, metastatic lesions, and solid pseudopapillary tumors (SPTs) can also present as cystic in appearance.
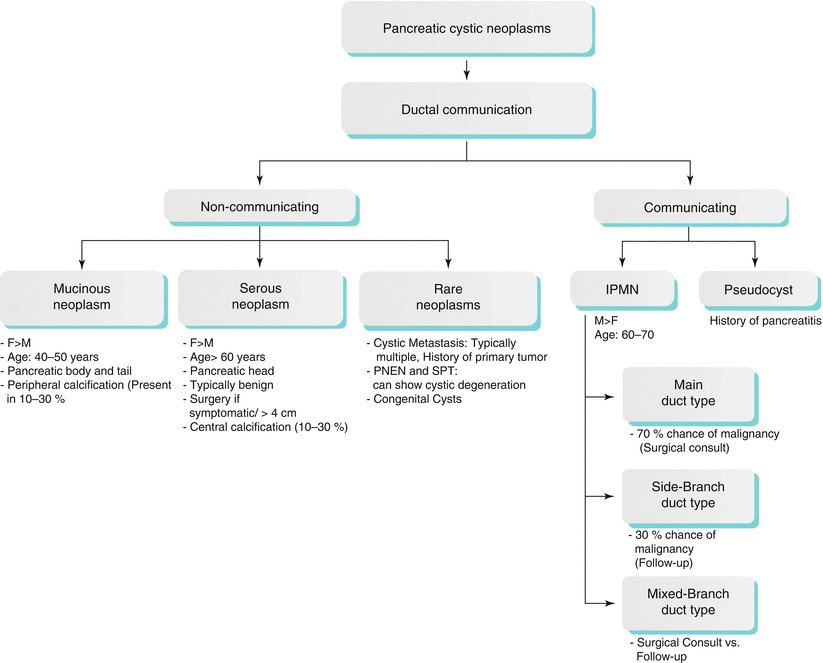

Algorithm 7.3 Diagnostic Approach of Pancreatic Cystic Lesions
CT and MR imaging are the mainstays of imaging. CT has better spatial resolution, while MRI has better contrast resolution and can better detect the relationship of the lesion to the pancreatic duct. ERCP with endoscopic ultrasound can be useful in settings of greater than 3 cm enlarging cystic lesions of the pancreas, for internal characterization, and for possible aspiration of cyst fluid [76, 77]. PET-CT can be useful for characterization of the cyst, as cystic lesions with invasiveness may show FDG uptake, specifically IPMNs [79, 80].
Mucinous Cystic Neoplasms
Mucinous nonneoplastic neoplasm (MNN) is very difficult to distinguish from other mucinous neoplasms on imaging. Histologically, they have an epithelial wall (unlike pseudocysts) and mucinous differentiation of the epithelial lining without surrounding ovarian stroma (unlike mucinous cystadenoma {MCA}), and there is no ductal communication, papillary projections, or cellular atypia (unlike IPMNs) [76, 81]. On CT and MR imaging, MNNs appear unilocular, without internal complexity except for occasional thin septations, which makes it very difficult to differentiate MNNs from MCAs. They do not have a gender predilection and do not communicate with the main pancreatic ducts. On the other hand, the MCAs have a female predilection and may or may not have a ductal communication, as these can fistulize with the duct [76, 77]. IPMNs are typically seen in older males, have ductal communication, and can present multiple occurrences in one patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree