- •
Noninvasive imaging plays an important role in the evaluation of patients with new-onset HF.
- •
Documentation of angiographic CAD is often a first-line test and is supported by guidelines.
- •
The presence of angiographic CAD does not establish a causal relationship between CAD and LV dysfunction.
- •
Demonstration of ischemia and/or scar with radionuclide MPI (or other techniques) plays an important role both in the diagnosis of ICM and in guiding management, especially the possible need for revascularization.
- •
FDG PET imaging is emerging as a complementary tool to CMR in the evaluation of myocardial inflammation in patients with suspected myocarditis. As in cardiac sarcoidosis, quantification of myocardial inflammation may also be used to monitor response to anti-inflammatory therapies.
- •
The potential role of quantitative MBF by PET for risk prediction and as a potential therapeutic target in patients with idiopathic DCM warrants further investigation.
Introduction
Heart failure (HF) is a clinical syndrome characterized by typical symptoms of breathlessness, ankle swelling, and fatigue. It may result from disorders of the pericardium, myocardium, endocardium, or heart valves, but because most patients have symptoms related to impaired left ventricular (LV) myocardial function, we will focus mainly on clinical scenarios characterized by abnormalities of systolic and/or diastolic LV function.
In patients with HF symptoms, the main classification is based on a measurement of the LV ejection fraction (LVEF) generally performed by echocardiography. Radionuclide ventriculography or magnetic resonance imaging (MRI) can be useful to assess LVEF and volumes when echocardiography is inadequate. HF in patients with LVEF equal to or greater than 50% is defined as HF preserved ejection fraction (HFpEF), whereas in patients with LVEF equal to or less than 40%, it is defined as HF reduced ejection fraction (HFrEF). The intermediate LVEF range represents a “gray area,” which is referred to as HF midrange ejection fraction (HFmrEF). Differentiation of patients with HF based on LVEF is important because it helps guide identification of its etiology and define management. Compared with HFrEF, patients with HFpEF are older, more often women, and more often have a history of hypertension and atrial fibrillation, but a history of myocardial infarction (MI) is less common. Biochemical and imaging findings support the initial diagnosis of HF, including elevated levels of natriuretic peptide and objective evidence of cardiac structural (including left atrial enlargement or increased LV mass) or functional abnormalities associated with systolic and/or diastolic ventricular dysfunction.
Practice guidelines from the American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC) provide clinicians with an evidence-based approach to the evaluation of patients with acute or HF. These guidelines are updated regularly, incorporating, when necessary, new evidence published in the interim. ACC/AHA and ESC guidelines have many similarities ; differences will also be mentioned when relevant.
The initial diagnosis and management of HF relies heavily on cardiac imaging. Because of the rapid evolution of imaging technologies and multimodality options, professional societies have developed appropriate use criteria (AUC) to assist clinicians with the choice of imaging approaches in different clinical scenarios. The ACC/AHA and the ESC/European Association of Cardiovascular Imaging (EACVI) AUC for multimodality imaging are based on available evidence and, when evidence is lacking, on expert and national society consensus opinion. ,
New-onset heart failure
New-onset (so-called de novo) HF may present acutely (e.g., as a consequence of acute myocardial infarction [AMI]) or subacutely e.g., gradually, as in patients with dilated cardiomyopathy, who often have symptoms for weeks or months before the diagnosis becomes clear). Symptoms and signs of new-onset HF, as well as the underlying cause, may resolve with treatment (e.g., acute myocarditis, takotsubo cardiomyopathy). On the other hand, the underlying cardiac cause may not resolve (e.g., amyloidosis) or may resolve partially (e.g., treated coronary artery disease [CAD]), potentially causing recurrent symptoms. Other conditions, such as idiopathic dilated cardiomyopathy (DCM), may show substantial or even complete recovery with therapy or may further progress to end-stage HF.
Thus the demonstration of the underlying cause of new-onset HF is central for appropriate early management of these patients to improve prognosis. The cardiac cause of HF is usually a myocardial abnormality producing systolic and/or diastolic ventricular dysfunction because of an ischemic (obstructive and/or functional/microvascular coronary disease) or nonischemic etiology (primary/genetic, inflammatory, infiltrative myocardial disease). Nevertheless, abnormalities of the valves, pericardium, endocardium, heart rhythm, and conduction system can also cause HF, and more than one abnormality can be present.
In this chapter, we will discuss the applications of radionuclide imaging in the evaluation of patients with new-onset HF. Although the focus will be on the role of radionuclide imaging, we will also highlight applications of other imaging techniques. We will use case vignettes to illustrate how imaging can be used to answer fundamental questions in the patient with new-onset HF.
Patient-centered applications of multimodality imaging in patients with new-onset heart failure
Case vignette 1: A patient with heart failure and angiographic coronary artery disease without evidence of ischemia or scar
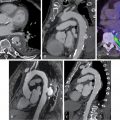
An 82-year-old male with exceptional functional capacity for his age (he played 18 holes of golf and hiked regularly) was admitted with acute pulmonary edema. Two-dimensional (2D) echocardiography showed severe and diffuse LV systolic dysfunction with an LVEF of 20%, without significant valvular disease. Despite no known history of cardiac disease and given his age and new-onset LV dysfunction, coronary arteriography was performed, which revealed moderate three-vessel CAD ( Fig. 18.1 A).
He underwent a nitrate-enhanced, rest 99m technetium (Tc) sestamibi single photon emission computed tomography (SPECT) myocardial perfusion scan to assess for myocardial viability in anticipation of potential coronary revascularization. The study showed normal radiotracer uptake in all myocardial regions, which was interpreted as evidence of preserved myocardial viability. Plans for coronary artery bypass grafting (CABG) were deterred by a chest radiograph that showed extensive lung fibrosis, a residue from remote radiotherapy for lymphoma (see Fig. 18.1 B). At this point, an adenosine-stress 99m Tc sestamibi SPECT myocardial perfusion scan was obtained to help clarify the risk-benefit ratio of seemingly high-risk CABG. The stress SPECT perfusion images showed a conspicuous lack of myocardial ischemia (see Fig. 18.1 C), which was confirmed by a stress myocardial perfusion cardiac magnetic resonance (CMR) study ( ![]() ). Based on the presence of diffuse, severe LV systolic dysfunction without evidence of myocardial ischemia or infarction, a diagnosis of nonischemic cardiomyopathy coexisting with CAD was made. After his acute pulmonary edema resolved, the patient was discharged on guideline-directed medical therapy for CAD and HF. Based on his excellent functional capacity and absence of angina, coronary revascularization was not performed.
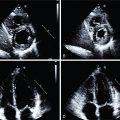
). Based on the presence of diffuse, severe LV systolic dysfunction without evidence of myocardial ischemia or infarction, a diagnosis of nonischemic cardiomyopathy coexisting with CAD was made. After his acute pulmonary edema resolved, the patient was discharged on guideline-directed medical therapy for CAD and HF. Based on his excellent functional capacity and absence of angina, coronary revascularization was not performed. ![]() shows an echocardiogram obtained 8 weeks after his index hospitalization demonstrating substantial recovery of LV systolic function.
shows an echocardiogram obtained 8 weeks after his index hospitalization demonstrating substantial recovery of LV systolic function.
Case vignette 2: A patient with heart failure and angiographic coronary artery disease with evidence of ischemia and scar
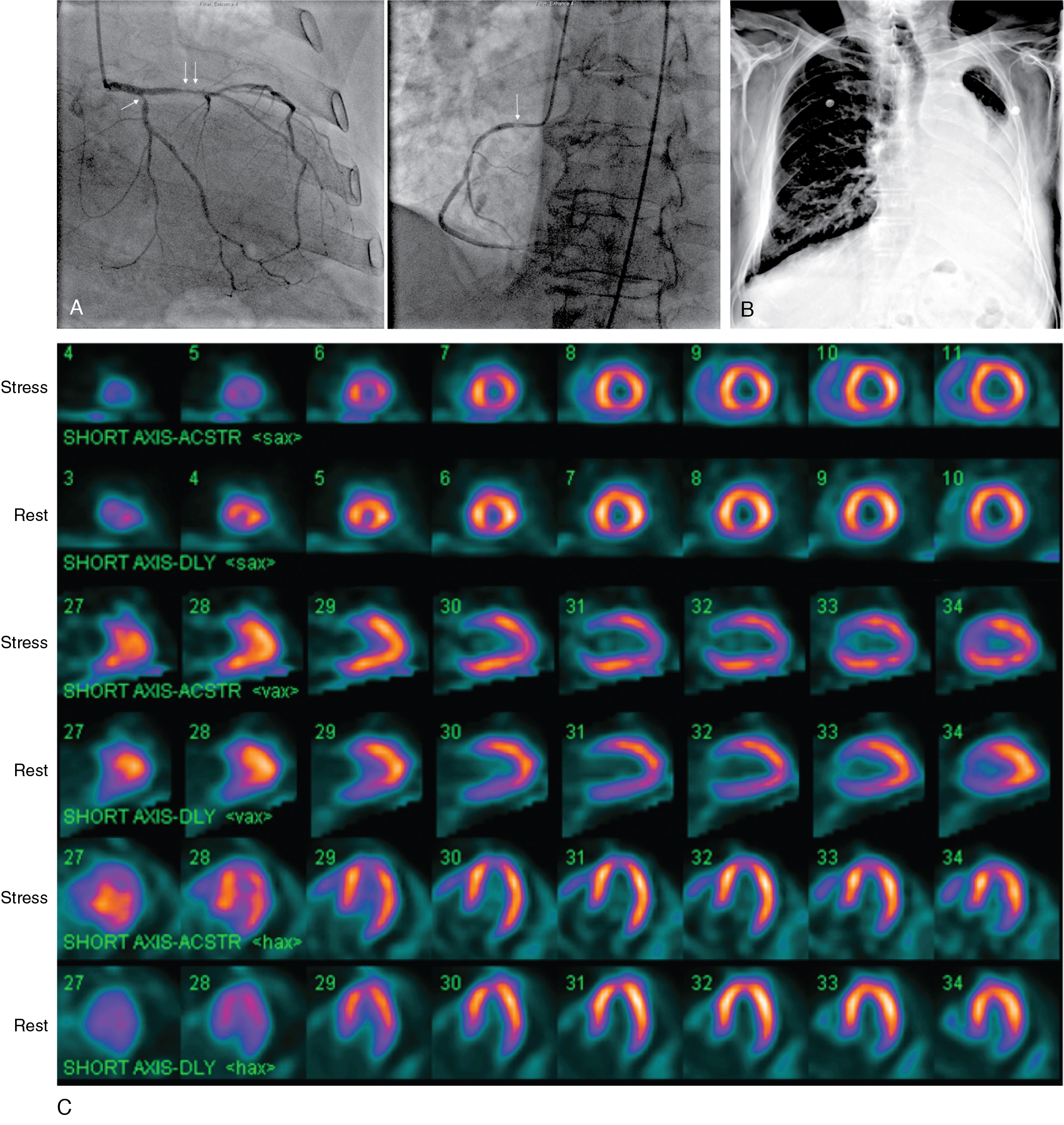
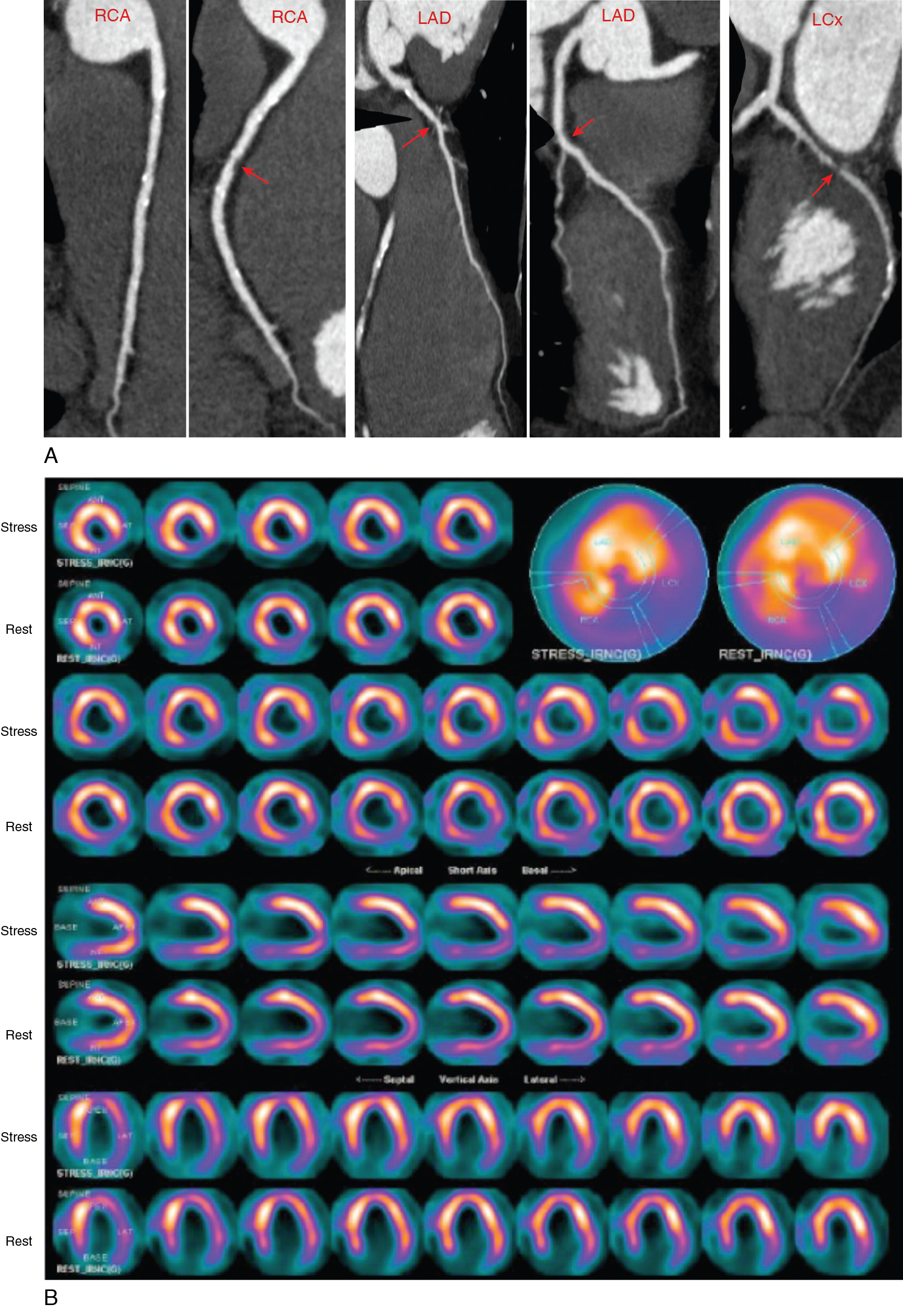
A 55-year-old man presented with mild dyspnea and palpitations. He had multiple cardiovascular risk factors, including current smoking, obesity, hypertension, and dyslipidemia treated with an angiotensin-converting enzyme (ACE) inhibitor, beta blocker, statin, and aspirin. The 12-lead electrocardiogram (ECG) showed frequent ventricular ectopic beats. A baseline 2D echocardiogram documented previously unknown LV systolic dysfunction with diffuse hypokinesia and severely depressed LVEF (30%). To confirm or exclude CAD as the underlying cause of new-onset LV dysfunction, a coronary CT angiography (CCTA) was performed that showed multiple mixed atherosclerotic plaques causing nonobstructive stenosis (<50%) in the middle portion of the right coronary artery (RCA) and the proximal left anterior descending artery (LAD) and a moderately obstructive stenosis (>70%) in the middle trait of the circumflex artery (LCx; Fig. 18.2 A and ![]() ). Because the extent and severity of angiographic CAD did not seem coherent with the degree of new-onset LV dysfunction, a functional evaluation with exercise stress SPECT myocardial perfusion imaging (MPI) was obtained to define the presence and extent of inducible ischemia. This was performed using an upright cycling ergometer during which the patient developed mild dyspnea but no chest pain or significant ECG changes at peak exercise. The stress MPI demonstrated a large and severe perfusion defect throughout the inferolateral and basal inferior walls and medium-sized perfusion defects in the anteroseptal wall and the LV apex, showing significant reversibility (ischemia: ∼19% of LV, scar: ∼7% of LV; see Fig. 18.2 B). Invasive coronary angiography demonstrated more severe CAD, compared with CCTA, with obstructive stenosis of the LCx artery (90%), intermediate stenosis (>50%) of the LAD, which was determined to be hemodynamically significant (fractional flow reserve [FFR] = 0.77), and a nonobstructive RCA stenosis (see Fig. 18.2 C). Based on the combined angiographic (CCTA and invasive angiography) and functional (SPECT and FFR) findings, ischemia from obstructive CAD was judged as the most probable underlying cause of his new-onset HF. The patient underwent successful percutaneous coronary intervention and stenting of the LCx and LAD coronary arteries (see Fig. 18.2 C).
). Because the extent and severity of angiographic CAD did not seem coherent with the degree of new-onset LV dysfunction, a functional evaluation with exercise stress SPECT myocardial perfusion imaging (MPI) was obtained to define the presence and extent of inducible ischemia. This was performed using an upright cycling ergometer during which the patient developed mild dyspnea but no chest pain or significant ECG changes at peak exercise. The stress MPI demonstrated a large and severe perfusion defect throughout the inferolateral and basal inferior walls and medium-sized perfusion defects in the anteroseptal wall and the LV apex, showing significant reversibility (ischemia: ∼19% of LV, scar: ∼7% of LV; see Fig. 18.2 B). Invasive coronary angiography demonstrated more severe CAD, compared with CCTA, with obstructive stenosis of the LCx artery (90%), intermediate stenosis (>50%) of the LAD, which was determined to be hemodynamically significant (fractional flow reserve [FFR] = 0.77), and a nonobstructive RCA stenosis (see Fig. 18.2 C). Based on the combined angiographic (CCTA and invasive angiography) and functional (SPECT and FFR) findings, ischemia from obstructive CAD was judged as the most probable underlying cause of his new-onset HF. The patient underwent successful percutaneous coronary intervention and stenting of the LCx and LAD coronary arteries (see Fig. 18.2 C).

Case Vignettes 1 and 2 illustrate the clinical importance of defining the predominant mechanism of LV systolic dysfunction, even in patients with angiographically obstructive CAD. Case Vignette 1 showed severe global LV systolic dysfunction and multivessel angiographic CAD but no evidence of myocardial ischemia or scar, consistent with nonischemic cardiomyopathy associated with CAD. In contrast, Case Vignette 2 showed congruent obstructive CAD and large areas of admixed ischemia and nontransmural scar, consistent with ischemic cardiomyopathy. The demonstration and quantification of scar and/or ischemia is a critical complement of coronary angiography for the correct interpretation of the predominant underlying mechanism of newly discovered LV systolic dysfunction to guide subsequent patient management.
Clinical trial and epidemiologic data indicate that 60% to 70% of patients with LV systolic dysfunction have CAD. , Although these data highlight the fact that CAD has overtaken hypertension as the most common etiology of heart failure, some inherent nuances in the attribution of HF etiology must be recognized. Taken literally, the term ischemic cardiomyopathy (ICM) appears to invoke a specific pathogenetic mechanism of LV systolic dysfunction involving inducible myocardial ischemia or prior infarction. In practice, it most often refers to LV systolic dysfunction associated with CAD without the necessary documentation of myocardial scar or ischemia; however, the relationship between CAD and LV systolic dysfunction could be one of coexistence (as in Case Vignette 1) rather than causality (as in Case Vignette 2).
This distinction between CAD that is etiologically related to the LV systolic dysfunction (ischemic cardiomyopathy) and that coexists with a nonischemic cardiomyopathy is important for reasons beyond semantic accuracy. The prognostic implications of causal versus coexisting CAD in patients with LV systolic dysfunction are vastly different. Therapeutic considerations of coronary revascularization are necessarily presaged by prognostic implications and thus must also be different between these groups. Felker and colleagues demonstrated this principle in an observational study of 1921 HF patients from the Duke Heart Failure Database, classified by the number of coronary arteries with obstructive CAD. After approximately 36 months of follow-up, the mortality of patients with single-vessel disease was no different from those with normal coronary arteries and significantly better than patients with more extensive CAD. The similar prognosis of patients with normal coronaries and limited-extent disease surely contests the benefit of coronary revascularization in the latter group, especially in the absence of anginal symptoms. Felker et al. proposed a revised definition of ischemic cardiomyopathy based on the presence of left main or proximal left anterior descending (LAD) coronary artery stenosis, at least two-vessel disease with equal to or greater than 70% stenosis or single-vessel disease with prior MI or coronary revascularization.
For patients presenting with new-onset LV systolic failure and anginal symptoms, practice guidelines uniformly recommend coronary angiography. This recommendation is based on the high prevalence of CAD in this population and the potentially curative effects of coronary revascularization on LV systolic dysfunction in patients with ICM and preserved myocardial viability. For patients with new LV systolic dysfunction without anginal symptoms, however, the guidelines are disparate in their recommendation for initial invasive versus noninvasive diagnostic testing. The ACC/AHA heart failure guidelines (2009) offer a Class IIA and IIB recommendation for CCTA and noninvasive imaging, respectively, and the ACC/AHA/American Society of Nuclear Cardiology guidelines for the use of radionuclide imaging (2003) suggest a Class IIA recommendation for MPI for this clinical situation. , Thus, there is heterogeneity in the approach to the patient with new-onset LV systolic dysfunction without angina or high risk for CAD, specifically with regard to the use of diagnostic CCTA or noninvasive imaging for initial testing.
The role of noninvasive imaging tests for differentiating ischemic and nonischemic cardiomyopathy has been investigated, using radionuclide imaging, echocardiography, CCTA, and CMR. Early radionuclide myocardial perfusion studies examining patients with established LV systolic dysfunction predated contemporary imaging methodology, using, for example, planar 201 thallium (Tl) imaging, and still had high sensitivity for CAD detection in this population. More recent studies using gated SPECT technology and 99m Tc-labeled radiotracers show that the presence and extent of perfusion defect and the regionality of LV dysfunction are predictive of significant CAD. The Investigation of Myocardial Gated SPECT Imaging (IMAGING) in Heart Failure trial specifically addressed the utility of gated SPECT MPI as an initial diagnostic strategy in patients hospitalized with new-onset HF. Two hundred and one such patients were prospectively enrolled and underwent exercise or pharmacologic SPECT during the index hospitalization. At the physician’s discretion, approximately one-third of the patients underwent CCTA. Using a summed stress score (SSS) greater than 3 to define an abnormal study, SPECT had a sensitivity of 96% and a negative predictive value of 96% for the diagnosis of ischemic cardiomyopathy using the criteria provided by Felker but was less accurate in detecting more limited CAD. These studies indicate that patients with LV systolic dysfunction and normal stress myocardial perfusion are unlikely to have causally related, extensive CAD. When angiographically obstructive CAD is detected in these patients, it is likely to coexist with a nonischemic process rather than be causally related.
The performance characteristics of radionuclide MPI are in keeping with the finite number of pathogenetic mechanisms by which CAD causes LV systolic dysfunction, namely scar (with or without remodeling), stunning, or hibernation. Therefore a normal stress MPI scan (or stress echocardiogram or CMR) in a low-risk patient without anginal symptoms effectively excludes CAD as the primary etiology of LV systolic dysfunction. This concept also applies to patients with chronic HF and is discussed in detailed in Chapter 20 . The presence of balanced ischemia stemming from extensive CAD or diffuse microvascular disease may rarely result in seemingly normal myocardial perfusion in the presence of true CAD-related LV systolic dysfunction, but evolving methods of positron emission tomography (PET) and SPECT myocardial flow quantification may help identify this state. , Dobutamine stress echocardiography may be less prone to this rare phenomenon. Furthermore, clinical experience suggests that it is unusual for extensive CAD, which is causative for LV systolic dysfunction, to exist without some indication in the patient’s symptomatology, risk profile, or ECG, and without abnormalities in resting perfusion or regional function. Nevertheless, many patients with newly recognized LV systolic dysfunction, even those at low clinical risk for ICM, will undergo coronary angiography.
This strategy of demonstrating one of the pathophysiological states of CAD-related LV systolic dysfunction as the basis for the diagnosis of ischemic cardiomyopathy is also important in myocardial viability imaging. As illustrated by Case Vignette 1, a normal resting myocardial perfusion establishes the presence of “viability” in dysfunctional myocardium but does not exclude a myopathic state as the basis for LV systolic dysfunction. In such patients, revascularization of coexisting CAD may not result in recovery of LV systolic dysfunction. On the other hand, the presence of myocardial ischemia on stress imaging or myocardial hibernation (combination of reduced resting flow and preserved glucose metabolism on PET) definitively establishes a role for ischemia in the etiology of LV systolic dysfunction and predicts a high probability of post-revascularization recovery of function ( Fig. 18.3 ).