Fig. 29.1
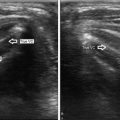
Liquid-based slide prepared on Thinprep machine and stained with Papanicolaou stain . The single slide provides a representative monolayer of the aspirated material
Some liquid specimens can be used for a cytospin preparation. This method concentrates the liquid sample and a portion is placed in a cyto-funnel on a cyto-centrifuge machine. This method produces a monolayer of cells within a well-defined area of the slide. Depending on the amount of the sample, multiple slides can be made for different types of stains, though the Papanicolaou stain is the most common. This method is most commonly used when the cellularity of a sample is scant (Fig. 29.2).


Fig. 29.2
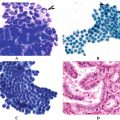
A Cytospin preparation stained with Papanicolaou stain after liquid fixative collection method. This method is often performed when the sample collected is small
Cell blocks are made from the formalin-fixed collection sample. The cell block can be prepared via several different methods, based on the techniques available to the specific lab. The sediment, clot, or tissue fragments are processed and embedded in paraffin. The paraffin block is cut on a microtome that makes thin tissue sections subsequently placed on glass slides. The end product is the traditionally hematoxylin and eosin (H&E) stained slides. The author’s laboratory does not routinely use cell blocks. In our experience this method does not add additional information unless metastatic carcinoma needs to be confirmed with immunohistochemical stains (Fig 29.3).


Fig. 29.3
A representative slide of hematoxylin and eosin stained cell block specimen. These samples are collected in a liquid fixative and paraffin embedded for cutting sections to place on slides
There are numerous methods for direct slide preparation. To prepare smears the aspirate is expelled onto the slide with the needle tip bevel down touching the glass to minimize spraying of the sample and possible air-dry artifact for any alcohol-fixed slides.
29.3.1 Classic Smear Technique
- 1.
Label frosted end with two patient identifiers (name, date of birth, medical record, etc.) using a #2 pencil
- 2.
Place a small drop of the aspirate a few millimeters from the frosted end
- 3.
Place another slide at the edge of the drop at a 45° angle and allow the sample drop to spread along the edge of the second slide
- 4.
Decrease the angle, then gently and quickly, push the specimen evenly away from the frosted end down the slide, trying to keep the smear at least 1 mm from the edges of the glass
- 5.
Immediately alcohol or spray fix one slide for Papanicolaou stain to be performed in the lab. The other slide can be air-dried and stained with a modified Romanowsky for ROSE or in the lab (Fig. 29.4a, b).

Fig. 29.4
(a) Properly prepared direct slide preparation with classic smears. The left slide is a Romanowsky stain stained smear and the right is a Papanicolaou stained smear that has the proper amount of specimen and keeps the smear edges at least 1 mm from the edges of the glass slide. (b) Improperly prepared direct slide preparation with classic smears. The left slide is a Romanowsky stain stained smear and the right is a Papanicolaou stained smear. Both slides have too much specimen causing the sample to cover the entire slide. The smearing pressure was uneven causing irregularities in the smear as well
Depending on the amount of material aspirated, multiple slides can be made. Optimally, one air-dried and one alcohol-fixed slide should be made from each pass. This allows for ROSE to be performed if desired and allows for evaluation of the specimen using both stains.
29.3.2 Bookend Smear Technique
- 1.
Label two slides at the frosted end with two patient identifiers (name, date of birth, medical record, etc.) using a #2 pencil.
- 2.
Place a small drop of the aspirate in the middle of one slide.
- 3.
Place the labeled, frosted side of the second slide face down onto the slide with the sample drop.
- 4.
Allow the sample to spread by capillary action, do not apply pressure.
- 5.
When the sample stops spreading, open the slides like a book, or pop them open. DO NOT SLIDE THEM ACROSS EACH OTHER.
- 6.
Get Clinical Tree app for offline access
This will produce two mirror image slides. Immediately alcohol or spray fix one slide for Papanicolaou stain to be performed in the lab. The other slide can be air-dried and stained with a modified Romanowsky for ROSE or in the lab (Fig. 29.5a, b).

Fig. 29.5
(a) Properly prepared direct slide preparation with bookends method. The left slide is a Romanowsky stain stained smear and the right is a Papanicolaou stained smear that has the proper amount of specimen and creates mirror image slides from one pass. (b) Improperly prepared direct slide preparation with bookends. The left slide is a Romanowsky stain stained smear and the right is a Papanicolaou stained smear. Both slides have too much specimen causing the sample to cover the entire slide. The cover slip does not sit well on the specimen due to the increased thickness of the sample. The thickness issue could have been fixed by applying an additional slide to remove the excess sample, thus providing additional better prepared slides
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree