Late gadolinium enhancement (LGE) has become a standard clinical tool to evaluate myocardial fibrosis to define myocardial viability in the context of ischemic myocardial disease. More recently, LGE has also been used to characterize the presence and pattern of fibrosis in nonischemic cardiomyopathies. It yields unique and valuable diagnostic and prognostic insights for myriad nonischemic clinical indications and has become a key part of routine cardiac MR imaging, and a tool to guide treatment. This article reviews the technical aspects of LGE performance and its diagnostic and prognostic implications in nonischemic cardiomyopathy.
Key points
- •
Cardiac magnetic resonance late gadolinium enhancement (LGE) is a robust imaging sequence that has become routine in the investigation of myocardial diseases.
- •
LGE is useful for diagnosis of the etiology in many myocardial diseases.
- •
The presence and extent of LGE have important prognostic implications in patients presenting with heart failure for most etiologies.
Technical aspects of late gadolinium enhancement
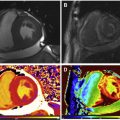
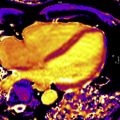
Gadolinium is a paramagnetic ion that has T1-shortening effects. When injected intravenously, it transits through the blood, into the myocardial extracellular volume (ECV), then washes out and recirculates to some degree before ultimately leaving the body unmetabolized via urinary excretion. Normal myocytes are tightly compacted with a small ECV, but pathologic myocardium affected by inflammation, infiltration, or infarction may expand the ECV, allowing for gadolinium accumulation upon its first passage through the myocardium ( Fig. 1 ). With appropriate timing appreciative of gadolinium kinetics and MR imaging hardware, including cardiac gating, and software, especially sequences that maximize T1-weighted signal to noise, healthy myocardium may be differentiated from cardiomyopathy involving expanded ECV, as with infiltration, inflammation, or infarction, via late gadolinium enhancement or LGE imaging ( Fig. 2 ).

In order to perform late gadolinium enhancement (LGE) imaging for myocardial tissue characterization, the most common techniques approved for clinical use today have not deviated significantly from the classic T1-weighted gradient echo inversion recovery sequence read out several minutes after gadolinium infusion as initially described. Although other contrast agents have been tested, standard gadolinium contrast agents are used in the vast majority of clinical cases. Similarly, the timing delay after gadolinium infusion may differ depending upon the MR imaging hardware and sequence, but typically ranges from 10 to 20 minutes after gadolinium infusion. The gadolinium is dosed by weight, which varies according to each specific product and may be infused as a rapid bolus or over several seconds, but should not be infused slowly over many minutes in order to avoid gadolinium washout before the entire contrast dose has been administered. The gadolinium administration intended for LGE purposes can also be used to perform some clinically useful first-pass imaging acquisition during the infusion. Often this might be vasodilator stress and rest perfusion imaging, which complement infarct imaging, especially for patients with symptoms of possible angina or being considered for coronary revascularization. Other options to take advantage of the gadolinium infusion might include coronary or thoracic angiography or selective slice plane imaging of a cardiac mass for first-pass perfusion. The CE-MARC trial evaluated the incremental value of routinely performing coronary magnetic resonance angiography (MRA) with the gadolinium infusion in addition to vasodilator stress perfusion CMR. However, the coronary MRA did not add diagnostic value to perfusion CMR in CE-MARC, and the MRA requires navigator gating to correct for respiratory motion and so is relatively time consuming (5 to 20 minutes) on contemporary scanners. Therefore, most sites do not routinely perform coronary MRA when conducting stress perfusion CMR with LGE viability for clinical indications.
Cardiac MR imaging centers most commonly rely upon 2-dimensional short axis slices through the ventricular myocardium for LGE imaging. Longitudinal slices, such as 4 chambers, 2 chambers, or 3 chambers, are often obtained either in single slices or stacks through each plane. Typically, the inversion time and field of view are optimized for left ventricular (LV) myocardial imaging. Classically, the standard protocol has involved the MR imaging technician starting with a standard inversion time, or TI, such as 200 milliseconds depending upon the particular magnet and field strength, and then adjusting the TI at small increments such as 30 milliseconds to reduce artifacts and optimize signal to noise. A semiautomated approach involves a series of snapshots at incremental TI, called a look locker. The technician simply selects the optimal TI from the look locker sequence and proceeds with the remainder of the LGE imaging. The right ventricle may also be evaluated, but attention must be paid to ensure that the inversion time is correct, as the right ventricle inversion time may be 50 to 100 milliseconds different from the LV inversion time, although newer scanners can overcome this difficulty automatically. For example, phase-sensitive inversion recovery (PSIR) sequences available on new MR imaging hardware and software essentially eliminate the need to adjust TI or use a look locker and provide a faster, more accurate, and simpler approach to LGE imaging. However, PSIR LGE images still require review in order to ensure no images need to be repeated because of artifacts.
Alternative sequences have been performed and include steady-state free precession, or SSFP, readouts. In addition, newer MR imaging scanners are capable of performing free-breathing 3-dimensional LGE imaging of the whole heart using navigator techniques to eliminate respiratory motion, although the acquisition may require 3 to 10 minutes, depending upon the equipment. More recently, a black blood LGE technique has been reported to be more sensitive at differentiating subendocardial infarcts from that blood pool that has typically been bright on classic LGE imaging, which may obscure small infarcts and unfortunately results in false-negative LGE images.
Gadolinium contrast agents for LGE imaging in general are widely regarded as safe and low risk. Because gadolinium is a toxic heavy metal ion, clinical contrast agents chelate the gadolinium that is excreted in the urine. Nephrogenic systemic fibrosis (NSF), also known as nephrogenic fibrosing dermopathy, is a painful and debilitating chronic skin disease that has been rarely reported after gadolinium use for MR imaging. However, NSF has been almost exclusively reported in patients with glomerular filtration rates below 30 mL/s/m 2 , particularly patients on dialysis. Therefore, the US Food and Drug Administration (FDA) generally prohibits clinical use of gadolinium for those patients. Also, most cases of NSF were reported in association with gadolinium compounds with a linear molecular structure in contrast to newer agents with a cyclic molecule, which are safer. Gadolinium does cross the placenta and should not be administered to pregnant patients because of the risk of fetal toxicity. Rarely, anaphylaxis may occur. The American College of Radiology has published a summary of contrast agent safety that addresses these important topics.
Diagnostic implications of late gadolinium enhancement in nonischemic cardiomyopathy
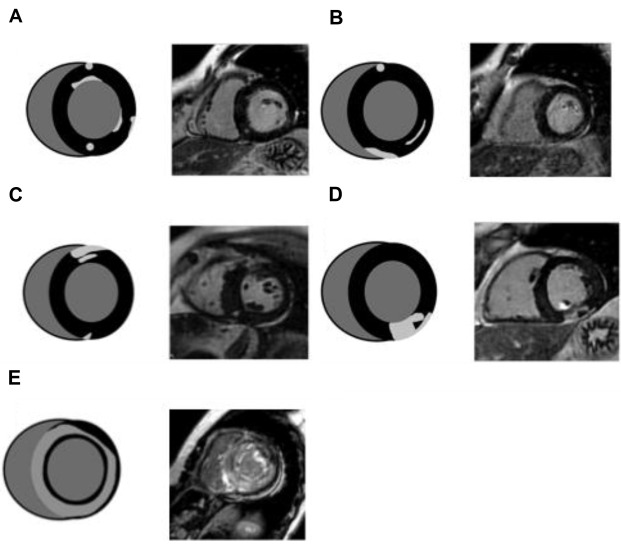
CMR is an extremely valuable tool for the evaluation of patients with cardiomyopathy and has an important impact on diagnosis, prognosis, and therapeutic decision-making. CMR plays a major role in determining the etiology of the underlying cardiomyopathy through its ability to characterize the myocardial tissue by using different techniques. Although this is usually performed with a combination of CMR sequences, tissue characterization generally includes late LGE, as it has been validated against histologic findings in a wide range of clinical scenarios. In particular, the pattern of LGE in the myocardium can help differentiate underlying etiology of nonischemic cardiomyopathies. An example of the impact of CMR data on the diagnosis of dilated cardiomyopathy is presented in Fig. 3 . A detailed list of diagnostic and prognostic implications of CMR is presented in Table 1 .

| Condition | Typical Pattern of LGE | Diagnosis | Prognosis/Risk Stratification |
|---|---|---|---|
| Valvular disease | Heterogeneous: focal, subendocardial, midwall, or extensive diffuse; aortic stenosis LGE often anteroseptal; VHD with pulmonary hypertension LGE may affect RV septal insertion | + | + |
| Sarcoidosis | Patchy, multifocal, may be intensely bright because of granuloma formation, usually sparing subendocardium, anteroseptal LGE extending into RV free wall = hook sign | + | + |
| HCM | Patchy, midmyocardial, spares subendocardium, LGE in regions of wall thickening | + | + |
| Amyloidosis | Global transmural or subendocardial, difficulty nulling myocardium, atrial walls, and interatrial septum | + | + |
| Chagas | Heterogeneous, predominantly inferolateral, inferior, and apical LV segments | + | + |
| Muscular dystrophy | Subepicardial, primarily inferolateral wall | + | + |
| Systemic Sclerosis | Midwall, patchy or linear, basal and mid-LV segments | + | Uncertain |
| Lupus | Localized, midwall, primarily interventricular septum | + | Uncertain |
| Postinfectious | Subepicardial or midwall, inferior, lateral, and basal anteroseptal | + | + |
| ARVC | RV LGE, subepicardial inferior, and lateral LV wall | – | + |
| Postchemotherapy | Subepicardial linear LGE, lateral LV wall | + | Uncertain |
Dilated cardiomyopathy
Dilated cardiomyopathy likely represents an end-stage manifestation of multiple nonischemic disorders that can damage the myocardium. Approximately one-third of patients with dilated cardiomyopathy (DCM) will have evidence of replacement myocardial fibrosis by LGE in the midwall of the interventricular septum, which can be related to chronic healing phase of myocarditis. CMR including LGE is useful in defining the etiology of DCM. It can differentiate ischemic from nonischemic LGE, including nonischemic patterns that may indicate other causes as will be further detailed.
Interestingly, in a large study of patients with nonobstructive dilated cardiomyopathy, 13% displayed LGE in an infarct pattern, despite the absence of obstructive coronary artery disease. This ischemic pattern may be a nonischemic pathology mimicking infarction or the result of transient occlusion caused by a nonobstructive unstable plaque, coronary vasoconstriction, or other transient myocardial ischemia. Nevertheless, most CMR studies have demonstrated that LGE was a highly effective diagnostic tool, with a significant impact as a gatekeeper to coronary angiography by excluding major 3-vessel coronary artery disease (CAD) or left main disease in individuals with DCM.
Moreover, the presence of LGE is associated with abnormalities in contractility and serves as a potential substrate for re-entrant ventricular arrhythmia. The presence of LGE identifies a cohort of patients who do not respond as well to optimal medical therapy, a finding independent of other standard clinical parameters such as QRS complex duration and N-terminal pro–B-type natriuretic peptide levels. Based on this evidence, some authors have proposed the use of LGE as a criterion for advanced therapy such as ICD, although current guidelines do not support such strategy.
Myocarditis
Myocarditis commonly presents as an acute inflammatory disease. In this setting, the presence of LGE identifies acute inflammation, although later in the course of the disease LGE may be associated with areas of irreversible necrosis or fibrosis. Although the LGE distribution in myocarditis may be variable, the finding of LGE in the mid-wall and subepicardium of the left ventricle seems specific for viral myocarditis and has been validated against histology. Additionally, some studies suggest the LGE distribution might be related to the type of virus.
Older studies suggested that early postcontrast T1-weighted enhancement of the myocardium could be a marker of inflammation in myocarditis. In addition, T2-weighted imaging demonstrating myocardial edema can be a diagnostic sign. Thus, the Lake Louise consensus group in 2009 provided recommendations on the use of CMR in patients with suspected myocarditis suggesting that positivity of 2 of 3 techniques (early enhancement ratio, LGE, and/or increased T2 signal) may be an ideal diagnostic approach for myocarditis. Hence, combination of these techniques may be the best way to accurately identify acute myocarditis, although depending on the clinical presentation, LGE alone may suffice to confirm the diagnosis.
Additionally, some studies have suggested that the evaluation of myocarditis should also include T1 or T2 mapping. In a study of 61 patients with acute myocarditis and 67 patients with chronic myocarditis, diagnostic accuracy of native T1, that is, T1 mapping before contrast, was 99%, compared with 86% for LGE alone and 72% for increased T2-weighted signal for diagnosing acute myocarditis. In chronic myocarditis, LGE alone performed better than T1 mapping (94% vs 84% accuracy), but the combination of the 2 techniques significantly improved overall accuracy. Nevertheless, T1 and T2 mapping are not yet included in myocarditis imaging guidelines.
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is the most common genetic myocardial disease and an important cause of sudden cardiac death in young people and athletes. Although echocardiography remains as the initial test in suspected HCM, CMR provides a more comprehensive evaluation, including a more sensitive assessment of myocardial hypertrophy. However, the most meaningful use of CMR in HCM is the ability to identify and quantify myocardial fibrosis with the use of LGE in this patient population. LGE has been reported in 63% to 67% of patients with HCM. In those individuals, LGE represents areas of focal interstitial expansion caused by myocardial replacement fibrosis, and it typically presents with patchy enhancement involving myocardial walls with the greatest hypertrophy and at the right ventricular (RV) septal insertion sites.
amyloidosis
Cardiac amyloidosis (CA) is a rare infiltrative disorder in which abnormally folded amyloid proteins are deposited within the myocardium, and CMR can be a valuable tool for its detection. Presence of amyloid proteins in myocardial interstitium is associated with characteristic patterns of LGE, usually related to marked expansion of the ECV.
LGE may appear for CA patients as a widespread circumferential subendocardial enhancement most pronounced at the base and middle of the ventricle, matching the distribution of amyloid protein on histology. Validation against endomyocardial biopsy was performed in 33 patients with diastolic dysfunction, and features concerning for amyloid and diffuse subendocardial LGE had a specificity of nearly 95% for CA diagnosis. Although circumferential subendocardial LGE is present in the majority of patients with CA, alternative patterns of LGE such as midwall, subepicardial, and interatrial septum enhancement are also commonly encountered (see Fig. 2 ). It is important to note that the interpretation of LGE images in this population can often be challenging because of the diffuse nature of LGE and the inability to adequately null the myocardial signal because of prolonged and significant gadolinium deposition into the expanded ECV.
CMR can also be used to discriminate light chain (AL) amyloidosis from the transthyretin (ATTR) CA forms. Not only are the morphologic features generally different between AL and ATTR, as ATTR is more associated with severely thickened myocardium, but also a study comparing LGE findings in 46 patients with biopsy-proven AL and 51 with ATTR amyloidosis,= noted that LGE was much more extensive in ATTR, with 90% demonstrating transmural LGE compared with only 37% in AL. These investigators developed an LGE scoring system that differentiated the 2 types with 87% sensitivity and 96% specificity.
sarcoidosis
Sarcoidosis is a multiorgan inflammatory disorder characterized by noncaseating granulomatous infiltration. The diagnosis of cardiac sarcoidosis may be challenging and delayed, particularly in patients without known extracardiac sarcoidosis, which may be underdiagnosed. Because of these complexities several diagnostic guidelines have been proposed, including the one from the Japanese Ministry of Health in 2006, the United States National Institute of Health algorithm from 2014, the World Association for Sarcoidosis and Other Granulomatous Disorders (WASOG) recommendations for initial diagnosis of cardiac sarcoidosis, and the Heart Rhythm Society 2014 clinical algorithm for determining probable cardiac sarcoidosis, defined as greater than 50% likelihood of cardiac sarcoid.
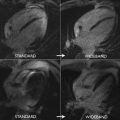
Challenges with all of the existing clinical diagnostic criteria include that history, physical examination, electrocardiogram (ECG), and echocardiography lack sensitivity and specificity; thus most imaging centers use either positron emission tomography/computed tomography (PET/CT) or CMR for evaluation of suspected cardiac sarcoidosis depending upon institutional expertise, with a trend toward CMR, as it involves no ionizing radiation and is not technically reliant upon patient glucose and insulin manipulation for diagnostic images. Both the WASOG and HRS criteria for sarcoidosis include CMR LGE, although the JMH criteria have not yet incorporated LGE. Several investigators have shown that CMR can readily identify individuals with CS by the identification of small areas of myocardial involvement using LGE. Furthermore, CMR and PET/CT offer complementary information such that both tests may be considered in some patients, as CMR best identifies area of fibrosis but is relatively incapable of identifying active inflammation or staging disease activity among sarcoid patients, as with PET-CT ( Fig. 4 ).

The LGE pattern in CS is variable, but it most commonly affects the basal to mid antero-septum with a classic pattern of midwall or epicardial enhancement. Often the LGE is focal and bright because of localized expansion of ECV with noncaseating granulomatous inflammation and fibrosis. Focal basal to midanteroseptal LGE with concomitant RV LGE has been termed the hook sign and is consistent usually with CS. Subendocardial or transmural enhancement in almost any coronary distribution can also be present, which may mimic infarction. One CMR study of 58 patients with biopsy-proven pulmonary sarcoidosis found 19 patients with evidence of LGE, mostly in the basal and lateral myocardium, a higher prevalence than identified by standard Japanese Ministry of Health (JMH) guidelines. Cardiac sarcoidosis is a protean disease, the evaluation of which benefits from careful integration of all clinical history and diagnostic studies with LGE CMR.
Arrhythmogenic ventricular cardiomyopathy
ARVC is an inherited cardiomyopathy that is associated with arrhythmias and sudden death. Because of its ability to accurately assess RV structure and function, MR imaging has become an integral part of the diagnosis of ARVC. The presence of RV LGE has been described in up to 60% of cases.
Diagnostic findings in this disease include RV dilatation and global or regional dysfunction including focal RV systolic dyskinesis or aneurysm. CMR is the gold standard method for the diagnosis of arrhythmogenic ventricular cardiomyopathy. Although in some cases, LGE of the RV free wall has been reported, this can be difficult to distinguish from myocardial fat. LV LGE, on the other hand can delineate different patterns of AVC. In the classic form, midwall LGE is seen in the inferolateral and inferior wall, while the left-dominant phenotype, often misdiagnosed as DCM, may include prominent LV midwall enhancement affecting the septum with preserved RV function. LGE is evaluated and often noted in patients undergoing evaluation for possible diagnosis of AVC, but RV LGE lacks accuracy and reliability. Thus, despite initial enthusiasm for RV LGE, this criterion was removed from the most recent AVD diagnostic guidelines in favor of RV volumes and systolic function.
Chagas
Chagas disease (CD) is a chronic disease caused by Trypanosoma cruzi infection and is an important cause of heart failure and mortality that predominantly affects Latin American countries. CD has 3 clinical phases: acute, indeterminate, and chronic. In its chronic form, CD can affect the gastrointestinal tract and/or the cardiovascular system. Chronic chagas cardiomyopathy (CCM) is both the most common and the deadliest manifestation of chronic infection. Although the clinical presentation and disease progression are highly variable, from minor rhythm disturbances to advanced heart failure, many of the complications associated with it are atrioventricular blocks and ventricular arrhythmias that can lead to sudden cardiac death (SCD). Myocardial delayed enhancement on cardiac MR imaging has surfaced as a diagnostic and prognostic tool for cardiac involvement in CD, as it has demonstrated higher sensitivity than electrocardiography and echocardiography. However, because the confirmation of CD is based on serologic evaluation, the role of LGE in the diagnosis of CD is mostly in the documentation of cardiac involvement from the systemic disease.
Other nonischemic cardiomyopathies
CMR is also able to diagnose a variety of other nonischemic cardiomyopathies, including takotsubo cardiomyopathy demonstrating myocardial edema without LGE, iron-overload cardiomyopathy (using T2* mapping), and Anderson-Fabry disease with characteristic findings of basal lateral LGE. Although LGE can be present in most myocardial diseases, the pattern of distribution might not be diagnostic in many, and its findings may solely be interpreted as an indication of structurally abnormal myocardium.
Prognostic implications of late gadolinium enhancement in nonischemic cardiomyopathy
Dilated Cardiomyopathy
Presence and extent of LGE in DCM have been linked to increased risk of SCD and ventricular arrhythmias independent of other markers of risk such as reduced LV ejection fraction (LVEF). In a prospective longitudinal study in 472 patients with dilated cardiomyopathy over a median follow-up of 5.3 years, patients with mid-wall fibrosis were significantly more likely to die (27% vs 11%) or have a significant arrhythmic event (30% vs 7%) when compared with patients without LGE. After adjustment for LVEF and other conventional prognostic factors, both the presence of fibrosis (HR, 2.43 [95% CI, 1.50–3.92]) and its extent (HR, 1.11 [95% CI, 1.06–1.16]) were independently and incrementally associated with mortality.
In a prospective study of 137 patients being considered for ICD placement, myocardial scarring detected by cardiac MR imaging was an independent predictor of adverse outcomes. In patients with LVEF greater than 30%, significant scarring (>5% left ventricle) identifies a high-risk cohort similar in risk to those with LVEF of no more than 30%. Conversely, in patients with LVEF of less than or equal to 30%, minimal or no scarring identifies a low-risk cohort similar to those with LVEF greater than 30%.
In another prospective study enrolling 399 patients with dilated cardiomyopathy and an LVEF greater than 40%, a midwall LGE pattern was present in 25% of individuals and was associated with a ninefold increase in the risk of SCD or aborted SCD when compared with patients without LGE. In this study, the estimated hazard ratios (HRs) for SCD or aborted SCD for patients with an LGE extent of 0% to 2.5%, 2.5% to 5%, and greater than 5% compared with those without LGE were 10.6 (95% confidence interval [CI], 3.9–29.4), 4.9 (95% CI, 1.3–18.9), and 11.8 (95% CI, 4.3–32.3), respectively.
Hypertrophic Cardiomyopathy
Several studies have examined the relationship between the presence of LGE and outcome in HCM. Among 243 patients with HCM over a follow-up period of 3 years, LGE was seen in 67% of patients, with an odds ratio (OR) of 5.5 for all-cause mortality and 8.0 for cardiac mortality. A meta-analysis with 2993 patients over a median follow-up of 3 years demonstrated that the presence of LGE was associated with a 3.4-fold increase in risk for SCD, a 1.8-fold increase in all-cause mortality, a 2.9-fold increase in cardiovascular mortality, and a trend for heart failure death. Given the high prevalence of LGE, its presence alone cannot be used as an indication for ICD. Interestingly, the extent of LGE may have more discriminatory value than its presence. In this meta-analysis, after adjusting for baseline characteristics, the extent of LGE was strongly associated with the risk of SCD with an HR of 1.36 for every 10% increase in LGE extent.
In another study with 1293 patients followed for 3.3 years, a continuous relationship was evident between LGE involvement by percent LV mass and SCD event risk in HCM patients, and an LGE of at least 15% of LV mass was associated with a twofold increase in SCD event risk in those patients otherwise considered to be at lower risk, with an estimated likelihood for SCD events of 6% at 5 years.
Valvular Heart Disease
Valvular heart disease is an important public health problem, and it is a growing concern with the increasing in prevalence in the aging population, particularly for aortic stenosis (AS). Although the current valvular guidelines recommend using CMR for evaluation of patients in whom echocardiography is not diagnostic, there is evidence that CMR can detect early signs of cardiac dysfunction and assist in risk stratification of patients with valvular diseases beyond usual care.
In patients with severe aortic valve disease, the chronic pressure or volume overload results in progressive myocardial injury, myocyte degeneration, and interstitial myocardial fibrosis. This was previously described in histopathological studies in patients with AS and aortic insufficiency (AI) who underwent valve replacement surgery. The process of myocyte degeneration and IM fibrosis was later correlated to decline in LV systolic function and heart failure. In patients with AS and AI, the amount of fibrosis correlates with increase in LV end diastolic volumes, end systolic volumes, and mass, as well as an inverse relationship with LVEF.
Weideman and colleagues were the first to evaluate how myocardial fibrosis on CMR relates to prognosis in patients with severe symptomatic AS. They evaluated 58 patients who were followed for 9 months after aortic valve replacement (AVR) with clinical evaluation and repeat imaging. In their cohort, the extent and severity of fibrosis measured by LGE correlated with baseline New York Heart Association (NYHA) functional class, as well as postoperative prognosis and function. After a 9-month follow up, the degree of LGE remained unchanged in all groups. Although patients with no LGE experienced a significant improvement in NYHA functional class, patients with severe LGE demonstrated no functional improvement. Additionally, mortality during follow-up occurred only in patients with severe LGE, suggesting that the fibrosis is irreversible and a marker of poor prognosis in this population. Dweck and colleagues also assessed the relationship of LGE and prognosis in patients with AS. They evaluated 143 patients with moderate to severe AS, followed them up for a mean of 2 years, and demonstrated that patients with a midwall LGE pattern, compared to matched patients with no LGE, had an eightfold increase in all-cause mortality. The risk of mortality also had a linear correlation with the LGE burden such that every 1% increase in LGE mass increased the risk of mortality by 5%. Although AVR improved survival in all patients, it did not lead to reversal of LGE, and the increased mortality associated with LGE persisted despite the intervention, with an incidence of 53.8 cases per 1000 patient-years in patients with LGE versus 13.7 cases per 1000 patient-years in patients without LGE. The association between presence of LGE and perioperative outcomes was also evaluated in the 63 patients who underwent AVR. Compared with no LGE, the presence of midwall LGE was associated with a significantly increased 30-day composite endpoint of death, myocardial infarction, and cerebrovascular accident (CVA), and this was largely driven by CVA. The patients with midwall pattern LGE also had 3 times higher rates of heart block and a trend toward higher rates of ventricular tachycardia compared to patients with no LGE. Survival in patients with severe AS and evidence of LGE undergoing surgical and transcatheter AVR was examined by Barone-Rochette and colleagues. In their cohort of 154 patients, 44 had preoperative evidence of LGE, which was associated with a significantly increased all-cause mortality and cardiovascular mortality over a median 2.9-year follow-up. In the largest cohort to date, Chin and colleagues evaluated the association of myocardial fibrosis and outcomes in patients with aortic stenosis in 166 patients with AS followed for 2.9 plus or minus 0.8 years. In their cohort, LGE was associated with more severe AS, increased LV mass, impaired LV performance, and impaired functional status. LGE was also associated with a significantly higher mortality versus individuals with no LGE, with 71 deaths per 1000 patient-years versus 8 deaths per 1000 patient years. Additionally, although there was an association between the degree of AS and presence of LGE, about one-third of the patients with LGE had moderate AS, with similar outcomes to those with severe AS, suggesting that LGE prognostic value is independent of the degree of stenosis.
Similar findings have been reported by Azevedo and colleagues in a study of 54 individuals, 28 with predominant AS and 26 with predominant AI, who were undergoing AVR. They obtained baseline and repeated CMR at 27 months and extended follow-up to 4.3 plus or minus 1.4 years. On follow-up imaging, there was no change in the degree of LGE following AVR. Patients with more extensive LGE had significantly less postoperative improvement in LV systolic function. Additionally, presence of LGE was associated with a 1.26 times higher risk of mortality in a multivariate analysis, suggesting once more an association between LGE.
In mitral regurgitation (MR), LV remodeling and myocardial fibrosis may occur before the development of symptoms. In a study that evaluated LGE in 41 patients with at least moderate asymptomatic primary MR, 31% of patients had evidence of LGE on MR imaging. LGE was present in both infarct and midwall patterns and correlated to increased LV end systolic and end diastolic volumes. Similar findings were seen in a cohort of 35 asymptomatic patients with at least moderate primary MR, 31% of whom were found to have midwall pattern LGE, which correlated with significantly increased left atrial volumes compared to patients without LGE. These findings suggest that LV remodeling can be detected by CMR prior to development of LV dysfunction and clinical symptoms. Han and colleagues evaluated patients with mitral valve prolapse (MVP) with CMR and found that 94% of their cohort had LGE on the mitral valve or the mitral annulus, while 63%, were found to have papillary muscle LGE, which strongly correlated to presence of complex ventricular arrhythmias, although this analysis was limited by a small number of events. In a more recent study of patients with MVP and moderate-to-severe MR, the authors found no correlation between presence of LGE and arrhythmias by symptoms or ambulatory monitoring in patients with positive papillary muscle LGE. With respect to LGE and perioperative prognosis, Chaikriangkrai and colleagues evaluated 48 patients with chronic severe primary and secondary MR undergoing mitral valve repair and followed them for a median of 11 months. They observed LGE in 40% of patients, which was associated with a 4.8-fold increased risk of permanent pacemaker implantation, repeat hospitalization, and ICU readmission.
Sarcoidosis
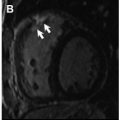
Over a median follow-up of 84 months, LGE on CMR was the only independent predictor of the composite endpoint of all-cause mortality, symptomatic life-threatening arrhythmia, unplanned hospitalization for heart failure, and cardiac transplantation HR: 5.7 (95% CI: 1.7–18.5) in a study of 312 individuals with proven sarcoidosis. A meta-analysis of prognostic CMR studies patients with known or suspected cardiac sarcoidosis demonstrated that the presence of LGE was associated with a 3.1% versus 0.6% annualized all-cause mortality (relative risk [RR]: 3.4, P =.04), 1.9% versus 0.3% cardiovascular mortality (RR: 10.7, P =.03), as well as ventricular arrhythmia occurring in 41 LGE-positive versus 0 LGE-negative patients (RR: 19.5, P =.003, Fig. 5 ). More of this topic will be discussed in Hélder Jorge Andrade Gomes and colleagues’ article, “ The Value of T1 Mapping Techniques in the Assessment of Myocardial Interstitial Fibrosis ,” in this issue of this text.