The Retroperitoneum
This chapter deals with those conditions that involve the urinary tract such as fluid collections, retroperitoneal fibrosis, and primary retroperitoneal tumors. It will not deal with the wide topic of lymphatic diseases; nodal metastases from urologic or gynecologic neoplasms will be dealt with in the chapters describing these tumors.
FLUID COLLECTIONS
Hemorrhage
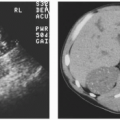
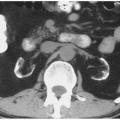
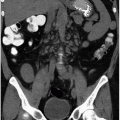
The causes of retroperitoneal hemorrhage are numerous; clinical and imaging information usually reveals the source, but sometimes the reason for bleeding is initially obscure. Interventional procedures (Fig. 4.1) and other trauma are common causes of hemorrhage. Coagulopathies and anticoagulation therapy may cause hemorrhage with no associated underlying pathology and exacerbate the tendency of a number of kinds of retroperitoneal pathology to undergo spontaneous bleeding. Any tumor may bleed; in the kidney, angiomyolipomas (Fig. 4.2) and carcinomas are the commonest causes. In the adrenal gland, pheochromocytomas (Fig. 4.3), metastases, and adrenocortical carcinomas may hemorrhage. Vascular diseases may cause bleeding; any aneurysm, including those of the abdominal aorta (Fig. 4.4) and the extrarenal and intrarenal segments of the renal artery, may rupture. Arteriovenous malformations and fistulae may bleed, as may vessels affected by arteritis (Fig. 4.5). Severe shock may cause hemorrhage in normal adrenal glands. The psoas and iliacus muscles are particularly prone to undergo bleeding (Fig. 4.6), which may appear with anticoagulation, after arterial catheterization or following relatively mild trauma.
If an intrarenal or a perirenal hematoma appears spontaneously, and no underlying cause is visible at initial workup, repeat imaging after hematoma resolution may be advisable to exclude a small tumor.
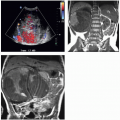
Small hemorrhages may occur without symptoms; larger ones may produce pain, shock, and/or anemia. Rarely, an evolving perinephric or subcapsular hematoma will exert sufficient pressure on the kidney to produce the Page kidney phenomenon (Fig. 4.7), in which pressure-induced renal ischemia activates the renin-angiotensin system and causes hypertension. Most hematomas ultimately resorb completely, but some persist. These become encapsulated and undergo resorption of hemoglobin, becoming chronic fluid collections known as seromas (Fig. 4.8).
CT is the first examination to employ if hemorrhage or hematomas are suspected. Noncontrast CT suffices to identify hematomas, but CT arteriography should be performed if active bleeding is suspected. MRI and MR arteriography are probably equivalent, but to date, there has not been extensive comparison of them in evaluating retroperitoneal bleeding, and CT is usually more quickly available, cheaper, and less prone to technically inadequate studies. Catheter arteriography may be necessary to demonstrate small vascular abnormalities, and permits embolotherapy.
Extravasated blood may infiltrate soft tissues to produce a contusion or may collect in a space containing only blood. Hematomas frequently appear in the perinephric space; anterior pararenal space hematomas may be caused by pancreatitis and pancreatic or duodenal trauma. Posterior pararenal space hematomas often originate
in the psoas or iliacus muscles (Fig. 4.6). Hematomas may displace any retroperitoneal viscus or blood vessel.
in the psoas or iliacus muscles (Fig. 4.6). Hematomas may displace any retroperitoneal viscus or blood vessel.
 FIGURE 4.1. Retroperitoneal hematoma following biopsy of transplanted kidney (not shown). The denser portions of the hematoma are sites of concentrated hemoglobin. |
 FIGURE 4.2. A: Large hematoma (arrows) surrounding spontaneously bleeding renal angiomyolipoma. B: The angiomyolipoma (arrows) several months prior to hemorrhage. |
 FIGURE 4.3. Cystic adrenal pheochromocytoma (black and white arrows) with spontaneous hemorrhage (white arrows). |
Ultrasound delineates fatty contusion poorly but will reveal most discrete hematomas as regions hypoechoic compared to fat but still containing disorganized echoes and usually displaying enhanced through-transmission (Fig. 4.9); chronic seromas usually have very few internal echoes. CT is highly sensitive in detecting hemorrhage and provides a clear delineation of its location and extent. Acute hematomas usually display feathery margins (Fig. 4.3) unless they are contained by a smooth fascial sheet. The CT density of blood is directly proportional to hemoglobin concentration, so that when clots form, or red cells settle in the dependent portion of the hematoma, dense regions appear. These will be denser than any noncalcified tissue (albeit <100 Hounsfield units) on unenhanced scans. Intramuscular hemorrhage may present without remarkable increases in density and appear as nonspecific swelling.
When studied by MRI, extremely acute hematomas generally exhibit low to medium signal intensity on T1-weighted images and brighter signal on T2-weighted images. As the hematoma ages, the periphery increases in signal intensity on T1-weighted images (Fig. 4.10), and in the chronic phase, there may be a region of uniform high intensity on both T1 and T2 images.
 FIGURE 4.5. Arteritis. The kidneys are inhomogeneously enhanced, and a hematoma (arrows) from a bleeding vessel surrounds the left kidney. |
 FIGURE 4.6. Noncontrast CT reveals hematoma as faint dense region (arrows) in enlarged right psoas muscle. |
If a hematoma is treated conservatively, it may completely resorb and leave no more than thin linear fibrotic remnants. Sometimes, the hemoglobin will be resorbed but a fluid collection, termed a seroma, will remain. On CT, the fluid will ultimately approach water density. MRI may reveal a hemosiderin ring (low signal on all sequences) with fluid that is low to moderate signal on T1-weighted images and high signal on T2-weighted images.
Urinoma
Urine may escape from the collecting system or ureter into the retroperitoneum. If the urine leak is transient and of low volume, the fluid is usually resorbed. But a large volume leak may appear as a fluid collection while it is being resorbed, and occasionally, such a collection becomes encapsulated and remains as a chronic cystic lesion. A urinoma may be subcapsular in location (Fig. 5.6) or confined to the perirenal or pararenal spaces. Leaking urine occasionally tracks into the peritoneal cavity and becomes urinary ascites. Subcapsular urinomas, like hematomas, may compress the renal parenchyma and cause hypertension via the Page kidney phenomenon.
 FIGURE 4.7. Perirenal hematoma producing Page kidney. Despite acute blood loss, patient became actively hypertensive. |
 FIGURE 4.8. Subcapsular hematoma. The flattened surface of the kidney indicates that the fluid is within the renal capsule. The hemoglobin has been resorbed; the collection is now a seroma. |
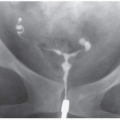
Urine leaks may be caused by trauma (Fig. 4.11), including surgery, that interrupts the walls of the collecting system or ureter or causes a renal parenchymal laceration that extends into the collecting system. Ureteral obstruction may also produce a urine leak: a healthy kidney may generate very high urine pressure proximal to a stone or other cause of acute severe ureteral occlusion, which in turn causes a rupture where the wall of the calyceal fornix meets the surface of the medullary papilla. Leaked urine (“pyelosinus extravasation”) then extends from the renal sinus through the renal hilum into the perinephric space (Fig. 4.12), where it may accumulate in a layer adjacent to the renal capsule, or flow along the outer surface of the ureter, sometimes forming urinomas. Leakage of sterile urine is seldom of concern, but, if infected, extravasated urine may produce perinephric abscesses.
 FIGURE 4.11. Chronic urinoma after trauma. The right kidney is distorted by a large fluid collection. |
Urinomas may be demonstrated by any cross-sectional imaging technique. Ultrasound will reveal an anechoic cystic region with enhanced through-transmission; the fluid will be of water attenuation on CT (Fig. 4.13) and have the signal characteristics of water (low signal on T1-weighted images, bright on T2-weighted images) on MRI. Acutely leaking urine may be opacified by excreted contrast if delayed images are obtained (Fig. 4.11).
Abscess
Retroperitoneal abscesses rarely occur spontaneously; they usually arise because of infection or perforation of a viscus within or adjacent to the retroperitoneum. Severe pyelonephritis, obstructive pyonephrosis, pancreatitis, spinal osteomyelitis and perforation of ulcers, and inflammatory or neoplastic disease of the ascending or descending colon, duodenum, or retrocecal appendix may all produce retroperitoneal abscesses. There is a tendency for duodenal and pancreatic disease to produce abscesses in the anterior pararenal space and for renal disease to cause abscesses in the perirenal space and osteomyelitis in the posterior pararenal space, but abscesses do not always respect the fascial planes that separate these compartments. Rarely, retroperitoneal fasciitis, which is like necrotizing fasciitis in any anatomic location, may be encountered.
Radiographically, abscesses may be identified since their walls are usually thicker and enhance more than those of sterile fluid collections (Figs. 4.14 and 4.15) and occasionally contain gas bubbles. These signs are not infallible: wall thickness and enhancement of infected and sterile collections may overlap, and air may appear from recent intervention and fistulae. There is often evidence of inflammation in adjacent tissue. Sonographically, pus is more likely than is sterile fluid to contain echo-producing debris; on CT, pus may be slightly denser than water, but absolute CT density is of little use in diagnosing infection.
Unless it is mixed with blood, pus has the same MRI characteristics as other fluid: low intensity on T1-weighted images and bright on T2-weighted images. CT is probably the best imaging technique for initial searches for retroperitoneal abscesses. Retroperitoneal fasciitis may produce CT evidence of inflammation, including fatty edema or “stranding,” fluid dissecting along fascial planes, and gas bubbles; these findings appear in a variety of conditions, but when encountered in a patient whose clinical picture is that of severe sepsis with pain and shock, retroperitoneal fasciitis should be considered.
Lymphocele
Lymphoceles are discrete collections of chylous fluid. They may occur as complications of pelvic or retroperitoneal surgery, particularly lymphadenectomy and renal transplantation. Typically presenting several weeks after surgery, they have been reported as occurring from as early as several days to as late as years after surgery. When quite large, lymphoceles may cause venous obstruction and lower extremity edema.
 FIGURE 4.15. Retroperitoneal abscess. T1-weighted gadolinium-enhanced image shows right posterior perirenal multichambered fluid collection with a thick enhancing wall. This arose from severe pyelonephritis, which, after antibiotic treatment, evolved to a renal abscess.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|