9 The Retroperitoneum
G. Krupski-Berdien, C.R. Habermann, and V. Nicolas
Introduction
In recent years MRI has evolved into a powerful imaging tool. The technical innovations that are most relevant in the setting of retroperitoneal disease include parallel imaging techniques and improved gradient echo sequences for 3D volume acquisition (e.g., VIBE).1 These advances have been made possible by the advent of high-performance gradient systems (30–40 mT/m), ultrafast sequences that can be acquired during breath-holding, and better techniques for magnetization preparation. Of increasing importance are optimized body coils and combinations of several surface coils for imaging of the entire retroperitoneum in a single acquisition (e.g., total imaging matrix— TIM, Siemens). Taken together, these new techniques enable full retroperitoneal evaluation in a short time while virtually eliminating artifacts. MRI is now the modality of choice for imaging benign soft-tissue tumors throughout the body because it enables evaluation of a set of malignancy criteria and identification of several specific tissues (e.g., well-differentiated liposarcoma). Further-more, MRI allows excellent definition of local tumor extent, providing crucial information for biopsy guidance and subsequent resection of tumors thought to be malignant on the basis of clinical behavior (e.g., growth) and imaging appearance. This information is important for predicting the prognosis of patients with soft-tissue tumors, which is mainly determined by the tumor-free resection margin and the histologic subtype.2
Precise evaluation of tumor extent in the retroperitoneum by MRI requires imaging in two planes, which should show the long and short axis of the tumor and take into account known patterns of spread of retroperitoneal soft-tissue tumors—these tend to grow in natural spaces such as muscle compartments and extend along anatomic pathways such as vessels or nerve sheaths.
Use of standardized imaging protocols is especially important in the retroperitoneum to ensure adequate interpretation of follow-up findings obtained on the same or a different MR scanner. In addition, MR images should show anatomic landmarks such as typical bone structures, joints, and unchanging soft-tissue structures (e.g., vessel bifurcations, muscles), which can help identify the site of a disease process on serial MR studies, especially after surgical resection.
This chapter discusses MRI of those retroperitoneal tumors and diseases that are not addressed in the organ-specific chapters.
Indications
MRI is indicated in patients with a retroperitoneal mass suggested by clinical findings, palpation, ultrasound, or CT or in patients with suspected diffuse disease such as retroperitoneal fibrosis. It is estimated that more than half of all retroperitoneal MRI examinations are performed for soft-tissue tumors. CT tends to be the preferred method in patients with inflammatory disease and abscess formation because it can also be used for interventional procedures. However, MRI is preferred for the evaluation of retroperitoneal fistulas, which may occur as a rare complication of Crohn disease.
Imaging Technique
All retroperitoneal MRI examinations are performed with the patient in the supine position. Intravenous access is established before imaging so that a contrast-enhanced dynamic study can be performed and an antispasmodic agent (butylscopolamine or glucagon) can be injected if necessary. In general, the retroperitoneum is imaged using a combination of spinal and abdominal phased-array surface coils. Phased-array multicoils can be combined with fast pulse sequences and parallel imaging techniques and are preferable to the whole-body resonator.3,4 Infants are often examined using a multichannel head coil, knee coil, or flexible surface coil.5
Imaging Planes
The examination usually begins with a coronal sequence to estimate the craniocaudal extent of a disease process and its topographic relationship to the iliopsoas muscle, the kidneys, and the adrenals. Axial images are useful for comparison with CT scans. The volume to be imaged and slice thickness are tailored to the specific clinical question. As a rule of thumb, a slice thickness of 4–10 mm (with no interslice gap) is chosen, depending on tumor size.
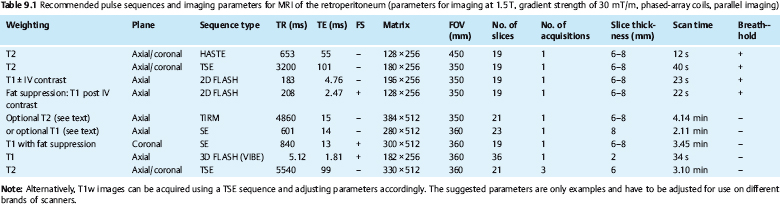
Pulse Sequences
A T2w HASTE sequence acquired during breath-hold allows rapid documentation of tumor extent in the retroperitoneum. Breath-hold imaging minimizes artifacts and will improve conformity with the prescribed slice locations. However, fast pulse sequences accentuate the fat signal compared with conventional sequences, making it more difficult to differentiate between fat and fluid. This problem can be overcome by using a fat suppression technique, which enables good separation of retroperitoneal fat and fluid (e.g., inversion recovery sequence or T2w sequence with spectral fat saturation).
Provided that the necessary equipment is available, T1w imaging before and after contrast administration should be performed with a gradient echo (GRE) sequence (e.g., 2D FLASH), which allows acquisition of larger slabs during a breath-hold of less than 30 s. GRE sequences are not only faster than spin echo (SE) sequences but also allow good identification of heterotopic ossification or hemorrhage as sources of artifacts distorting field homogeneity. Most indications for retroperitoneal MRI require intravenous contrast administration for improved delineation of tumor borders and internal architecture. For comparability, postcontrast imaging should be performed with the same parameters as the precontrast T1w series (Table 9.1). If a 2D FLASH sequence is used, it can be repeated with fat suppression after contrast administration, typically with acquisition of two phases. Alternatively, a 3D GRE sequence (VIBE) can be used, which combines fast acquisition with high resolution, but, owing to inherent fat saturation, provides no information on the presence of fat.
If T1w imaging during breath-hold is precluded for technical reasons, an SE or GRE sequence can be used, whereas T2w images should always be acquired with a fast SE (FSE or TSE) sequence. A T2*w sequence may be useful in patients with a rapidly growing tumor and suspected intralesional hemorrhage or to confirm a suspected hematoma. These pulse sequences can also be used for imaging below the iliac crest, where artifacts due to respiratory motion are negligible. Details of the imaging parameters are summarized in Table 9.1.
Contrast Media
An MRI examination for suspected retroperitoneal pathology is not complete without intravenous administration of contrast medium (e.g., Magnevist, Dotarem) at a standard dose of 0.1 mmol Gd per kg body weight. Newer contrast agents, in particular ultra-small superparamagnetic iron oxide particles (USPIO), have not yet proven beneficial in this setting, except for lymph node imaging.
Anatomy
The retroperitoneum is the connective tissue space behind the peritoneal cavity. Posteromedially, the lumbar spine and the psoas muscles on either side protrude into this space. Medially within the retroperitoneal space lie the abdominal aorta, the inferior vena cava, the ascending lumbar vein, portions of the sympathetic trunk, networks of vegetative nerves, and the chyle cistern. Lateral to the paired psoas muscle, the retroperitoneum widens to enclose the renal compartments. Through the posterolateral border of the retroperitoneum pass the lumbar plexus nerve, the subcostal nerve, and the segmental blood vessels and lymphatics. The retroperitoneum is bounded posterosuperiorly by the fascial sheath of the diaphragm and inferiorly by the peritoneal reflection onto the rectum and inguinal ligament. At its largest extent in the upper abdomen, the retroperitoneal space is subdivided into three compartments: the anterior pararenal space between the peritoneum and the anterior renal fascia (Gerota fascia), the perinephric space between the anterior renal fascia and the posterior renal fascia (Zuckerkandl fascia), and the posterior pararenal space between the posterior renal fascia and the fascial sheath of the autochthonous back muscles arising from the transversalis fascia. The fascia are very accurate anatomic boundaries but are not consistently visualized by MRI in healthy individuals, while they are often clearly delineated in the presence of inflammation.
Classification of Soft-Tissue Tumors
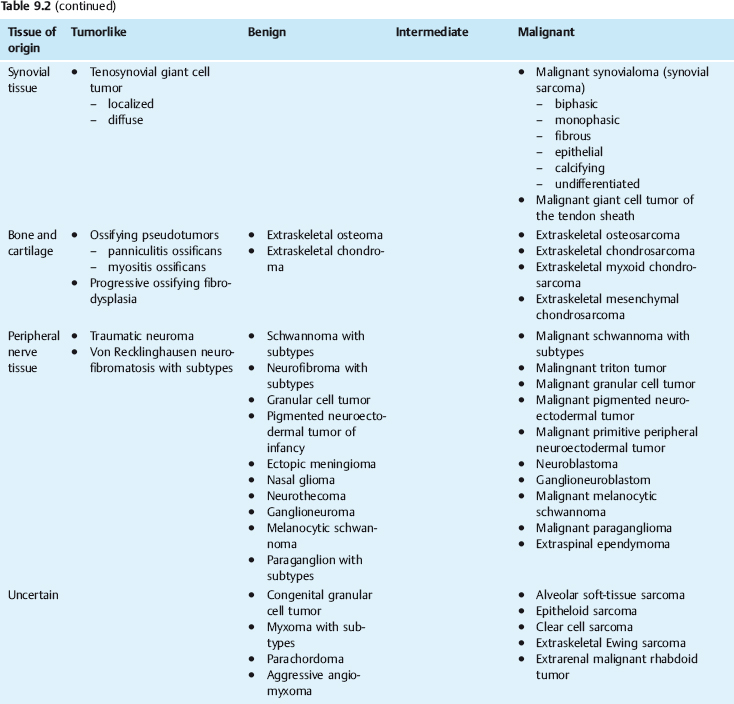
Soft-tissue tumors are neoplasms of mesenchymal origin that arise in the soft tissues of the extremities or in any other organ. Since connective tissue cells retain their ability to divide, these tumors can differentiate into various types of mesenchymal tissue elements (e.g., connective tissue, fat, muscle, cartilage, bone, nerve tissue, synovial tissue). The most widely used classification of soft-tissue tumors is the one by Enzinger and Weiss, 6 which is based on the different mesenchymal tissue types imitated by these tumors. This classification distinguishes benign, malignant, and indeterminate soft-tissue tumors on the basis of their biological behavior. A fourth class consists of tumorlike lesions, defined as nonneoplastic disease processes that mimic tumors. Like tumors, they can recur, but they have not been observed to metastasize. An example is deep fibromatosis. The very detailed classification presented in Table 9.2 provides an overview of the large group of soft-tissue tumors.
The staging classification of the American Joint Committee on Cancer, which is based on the TNM system, is most widely used in the clinical setting. The TNM staging system for soft-tissue sarcomas is presented in Table 9.3. The second important factor for therapeutic decisionmaking is the histologic tumor grade, which describes the degree of differentiation. Well-differentiated G1 tumors do not require adjuvant postoperative treatment if R0 resection has been achieved, but nearly all patients with dedifferentiated G3 tumors, which have a high recurrence rate, are candidates for postoperative radiotherapy and/or adjuvant chemotherapy.
Soft-tissue tumors are rare, and it is almost impossible to give exact frequency data. Especially for benign tumors, the incidence can only be estimated: a figure of ca. 300 per 100 000 individuals has ben proposed.6 Statistical data on malignant neoplasms are more reliable, since one may safely assume that all malignant soft-tissue tumors will come to clinical attention sooner or later and are then biopsied. For the USA, it has been estimated that a total of 486 000 malignant tumors occurred in1990, among them 5700 soft-tissue sarcomas with ca. 3100 sarcoma-related deaths. Based on these estimates, soft-tissue sarcomas constitute 1 % of all malignant tumors. The incidence reported in the literature is 1.35 to 1.4 per 100 000 population, 6 and it is assumed that the incidence of soft-tissue sarcomas of the extremities is higher in old age (up to 8 per 100 000 in those over 80).7 Important epidemiologic data are summarized in Table 9.4.
Some soft-tissue tumors have a predilection for specific sites—leiomyosarcomas constitute nearly half of all retroperitoneal, intra-abdominal, and visceral tumors. Next in frequency in the retroperitoneum are liposarcomas and schwannomas.4 Common tumors in the extremities are liposarcoma (26 %) and malignant fibrous histiocytoma (or the “newer” entities derived from these) (24%).
The classic histologic categorization (leiomyosarcoma, liposarcoma, malignant fibrous histiocytoma, etc.) is increasingly being abandoned in favor of a more refined classification based on immunohistochemical and molecular biological properties.8,9 At the same time these properties have the potential to serve as targets for new therapies: gastrointestinal stromal tumor (GIST), a variant of sarcoma, was established as a separate tumor entity quite recently. On the molecular level, it is characterized by partially excessive c-kit expression, making the tumor amenable to chemotherapeutic treatment with imatinab (Glivec/Gleevec), a tyrosine kinase inhibitor.
| T Primary tumor T1: Tumor ≤ 5.0 cm in greatest dimension T2: Tumor > 5.0 cm in greatest dimension |
| N Regional lymph nodes N0: No regional lymph node metastasis N1: Regional lymph node metastasis |
| M Distant metastasis M0: No distant metastasis M1: Distant metastasis |
| G Histologic grade of malignancy G1: Well differentiated (low malignancy grade) G2: Moderately differentiated (intermediate malignancy grade) G3: Poorly differentiated (high malignancy grade) G4: Undifferentiated (anaplastic) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree