|
Illustrate the normal gross and sectional anatomy of the scrotum and penis, including the vascular anatomy.
Describe the sonographic appearance of the normal scrotal and penile anatomy.
Explain the technique and protocol for sonographic evaluation of the scrotum and penis.
State the indications for a sonographic examination of the scrotum and penis.
Identify the common pathologic conditions that can result in an acute painful scrotum and penis.
Differentiate common extratesticular abnormalities from intratesticular abnormalities.
Describe the sonographic characteristics and laboratory values associated with scrotal masses.
Describe the sonographic characteristics associated with penile abnormalities.
alpha-fetoprotein levels are measured during pregnancy to detect certain fetal anomalies; blood levels may also be elevated with hepatocellular carcinoma and certain testicular cancers
human chorionic gonadotropin is produced during pregnancy and is also secreted by some malignant tumors, including certain testicular cancers
two cylindrical columns of spongy tissue running parallel dorsally that serve as the main erectile structures in the penis
single column of spongy tissue that contains the urethra and expands distally to form the glans penis; this tissue expands slightly during an erection but not to the extent of the cavernosa
undescended testicle—occurs when one or more of the testis fails to descend into the scrotum before birth
surgical procedure to remove a hydrocele
an increase in blood flow to the tissue
tissue death that occurs owing to a lack of blood flow
passage in the anterior abdominal wall in both females and males that transmits structures from the pelvis to the perineum
a surgical procedure done to fasten an undescended testicle into the scrotum or to repair an acute testicular torsion
a network of veins that drain the epididymis and testis; it is located in the spermatic cord and empties into the right and left testicular veins
characterized by fibrotic thickening of the tunica albuginea, resulting in the formation of fibrous plaques, which can lead to severe curvature of the penis and difficulty in achieving an erection
defined as a painful and prolonged penile erection, with or without stimulation
a sonographic indicator of an organ to perfusion; it is calculated from the peak systolic velocity and the end diastolic velocity of blood flow
scrotoliths or scrotal pearls are benign extra testicular macrocalcifications located within the scrotum, between the layers of the tunica vaginalis
process in which spermatozoa are produced
dense fibrous sheath that encapsulates and provides structure and support to the testicles and corpora of the penis
consists of forced expiration against a closed glottis after a full inspiration; the Valsalva maneuver increases the intra-abdominal pressure and is helpful in diagnosing a varicocele and scrotal hernia
surgical procedure in which the vas deferens is cut, tied, cauterized, or otherwise interrupted; the semen no longer contains sperm, preventing conception
a membranous sac attached to an embryo formed by cells adjacent to the embryonic disk
when evaluating large anatomic segments with inflammatory processes and fluid collections because a wider FOV provides a greater understanding of anatomical relationships10 (Fig. 19-2).
 FIGURE 19-1 Convex transducer. Transverse image of enlarged scrotum best seen with a convex probe. Note the large echogenic hydrocele compressing and displacing the testis (T). |
 FIGURE 19-2 Extended field of view (FOV). Longitudinal extended FOV image of the scrotum, including the testis and the epididymal head (H), body (B), and tail (T). |
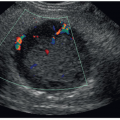
Obtain power, color, and spectral Doppler tracings to confirm the presence or absence of intratesticular arterial and venous flow.
Grayscale images are often nonspecific for evaluating testicular torsion and often appear normal when torsion is acute.16
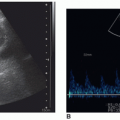
Spectral Doppler findings suggestive of partial torsion include asymmetry in resistive indices with decreased diastolic flow or diastolic flow reversal.16
In the clinical setting of epididymo-orchitis, when focal hypoechoic areas are detected within the testis, ultrasound follow-up is recommended after antibiotic treatment is completed to confirm the diagnosis and observe resolution, so that tumor and or infarction can be ruled out.7
Large hydroceles, hematomas, marked scrotal edema, and epididymo-orchitis are scrotal conditions that decrease intratesticular perfusion, mimicking testicular torsion.
In patients presenting with acute epididymo-orchitis, spectral Doppler demonstrates decreased vascular resistance (resistive index [RI] < 0.5) compared with the normal contralateral testis and epididymis.11
Reversal of the spectral diastolic component of the intratesticular arterial flow in patients with acute epididymo-orchitis suggests venous infarction.18
Use color and power Doppler to differentiate epididymitis from an enlarged, noninflammatory epididymis status postvasectomy.
Because primary varicoceles may decompress when the patient is supine, perform the Valsalva maneuver or scan the patient in the upright position to increase venous blood flow.
Large varicoceles can extend posteriorly, lateral, and inferiorly to the testis, mimicking epididymitis.
The inguinal canal and abdomen are imaged when hernia, secondary varicocele, undescended testis, and or postsurgical complications are suspected.
Evaluate the spermatic cord to detect abnormalities such as solid masses, hematomas, abscess, torsion, hernias, and hydroceles.
Use a gel standoff pad to evaluate anterior and/or superficial lesions, such as those in the tunica vaginalis.
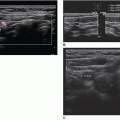
Longitudinal midline testis grayscale: Measure the length, and anteroposterior (AP) diameter. Midline testis: assess arterial and venous flow with color and/or power Doppler and color with spectral Doppler. Obtain an RI measurement of arterial flow.
Measure the AP diameter of the scrotal wall. Subsequent images should include the lateral and medial portions of the testis.
Longitudinal “cine-clip” of testis lateral to medial.
Longitudinal epididymal head grayscale: Measure the AP diameter and length.
Longitudinal epididymal head with color and/or power Doppler to assess vascular perfusion.
Longitudinal epididymal body: The normal narrow body of the epididymis lies adjacent to the posterolateral margin of the testis. Measure the AP diameter. Perform color and/or power Doppler imaging to assess vascular perfusion.
Longitudinal inferior testis and epididymal tail: Measure the superior to inferior diameter of the epididymal tail. Use color and/or power Doppler to assess vascular perfusion.
Longitudinal spermatic cord grayscale at rest and with the Valsalva maneuver and with color and/or power Doppler, to assess venous reflux and increased flow: Measure the AP diameter of the largest vein(s) on the grayscale image.
Transverse superior testis with epididymal head in grayscale and color to assess vascularity of epididymis compared with the testis.
Transverse mid-testis: Obtain transverse measurement in grayscale, and document with color Doppler. Subsequent images should include inferior testis and inferior testis with epididymal tail. Obtain grayscale and color Doppler to assess the vascularity of the epididymis compared with the testis.
Measure the AP diameter of the scrotal wall.
Transverse “cine-clip” testis superior to inferior.
Transverse spermatic cord grayscale at rest and with the Valsalva maneuver, and with color and/or power Doppler: Measure the AP diameter of the largest vein on the grayscale image.
with the testis.2,9,24,25 The narrow body and curved tail are smaller, more variable in position, usually posterior and inferior to the testis, and best evaluated in the longitudinal scan plane2,4,15,21,24,25 (Fig. 19-10B, C). Color flow imaging of the normal epididymis demonstrates speckled intraepididymal arterial flow7 (Fig. 19-10D).
scrotum.7 The scrotal ligament can be seen in the presence of a hydrocele. The sonographic appearance of the scrotal ligament is an echogenic band extending from the caudal end of the testis to the scrotal wall7 (Fig. 19-12).
has been identified unilaterally in 34% and bilaterally in 12% in postmortem studies.10,14 The appendix epididymis is approximately the same size as the appendix testis.10,11 The shape of the appendix epididymis is more of a stalk-like structure.10,11 Appendages of the testis and epididymis are visualized sonographically as isoechoic to echogenic protuberances superior to the testis and epididymis.7,10,11,14 Occasionally, the appendix epididymis may swell or distend, forming a cyst-like structure, not to be confused with an epididymal cyst10 (Fig. 19-16D).
 FIGURE 19-12 Scrotal ligaments. Longitudinal image of scrotal ligaments (SL, arrow) best seen in the presence of a hydrocele. Normal testis (T). |
 FIGURE 19-13 Tunica albuginea. Longitudinal image of testis demonstrating normal tunica albuginea (arrows). |
 FIGURE 19-15 Rete testis. Longitudinal image of normal testis showing echogenic stroma (arrow) with tubules of rete testis (RT). |
the cremasteric artery (a branch of the inferior epigastric artery).2,6,9,28 The deferential artery supplies the epididymis and vas deferens.2,6,7,9,11,28 Major blood supply to the epididymis, however, is via the superior epididymal artery, a branch of the testicular artery.6,11 The cremasteric artery supplies the peritesticular tissues (Fig. 19-18A, B). Both the deferential and cremasteric arteries also contribute a variable amount of blood to the testis via anastomoses with the testicular artery.2,7,9,28
 FIGURE 19-17 Testis. A: On the longitudinal of the normal testis, the calipers were used to measure length (1) and the width (2). B: Transverse image of normal testis. |
 FIGURE 19-18 Vascular anatomy. Schematic illustration of normal arterial supply to the scrotum (A), intrascrotal arterial supply (B), and venous drainage form the scrotum (C). |
In approximately 50% of normal testes, a transmediastinal arterial branch of the testicular artery enters the mediastinum and courses through the testicular parenchyma in a direction opposite to that of the centripetal arteries to supply the capsular artery. A transmediastinal vein usually accompanies the artery.5,7,9, 10 and 11,28,29 The grayscale sonographic appearance of the transmediastinal artery is a prominent hypoechoic band traversing the testis5,9 (Fig. 19-19A). With color Doppler, the transmediastinal artery is seen as a prominent arterial branch traversing the mediastinum, demonstrating flow toward the periphery of the testis to supply the capsular arteries5,7,10,38 (Fig. 19-19B). Flow in the transmediastinal artery courses in the opposite direction relative to the centripetal arteries5,6,28
TABLE 19-1 Sonographic Appearance of Common Scrotal Lesion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
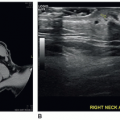
 FIGURE 19-21 Undescended testis. Longitudinal image of a small, undescended testis (arrows) located in the inguinal canal. MT, mediastinum testis. |
color or power Doppler to confirm the presence of arterial and venous flow within the testis because color Doppler can be subject to motion artifacts.13
 FIGURE 19-23 Types of testicular torsion. A: Schematic illustration of intravaginal testicular torsion. B: Schematic illustration of extravaginal testicular torsion. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree