Fig. 26.1
Anatomy of the testis and spermatic cord
Embryology
The gonads initially develop near the kidneys in the retroperitoneum. The formation of the gonad is dependent on three primordia: (1) primordial germ cells, (2) genital ridge, and (3) coelomic epithelium. The testes are suspended by the mesorchium, a double peritoneal fold. The upper fold transmits the spermatic vessels, and the lower fold forms the Hunter gubernaculum. The downward journey through the retroperitoneum commences at approximately the third month of gestation. During the seventh month the testes are found normally at the level of the anterior superior iliac spine. The peritoneum enters the inguinal canal ahead of the testes, but extends down the gubernaculum only part way. The testes and gubernaculum subsequently extend into the inguinal canal. The testes begin to enter the internal ring as the gubernaculum emerges from the external ring. As the gubernaculum reaches the bottom of the scrotal sac, it begins to shorten until its lower two-thirds have disappeared completely. At about the end of the seventh month, the testes pass through the inguinal canal. Although descent through the canal is accomplished in a few days, it takes four additional weeks for the testes to pass from the external ring to the bottom of the scrotum. Descent may be complete or may still be incomplete at birth. After the testes emerge through the external ring, the external ring contracts [3] (Fig. 26.2).
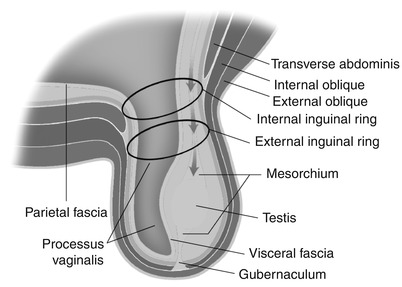

Fig. 26.2
Pathway of testicular descent and embryological makeup of the scrotum
Imaging Technique
When diagnosing disease processes that involve the scrotum and contents, ultrasound remains the primary modality of evaluation. Ultrasound has the advantages of general availability, portability, high resolution, functional information (blood flow), and relatively low cost. Limitations of the technique are its high dependency on the quality of the equipment and the expertise of the operator [4]. MR imaging is an excellent adjunct or problem solving tool for further evaluation of equivocal findings on ultrasound, complex clinical situations, or in patients where there is discordance between ultrasound findings and clinical presentation [5].
Ultrasound Imaging Technique
The examination should be performed in a warm room to avoid a cremasteric reflex (more pronounced in children), which pulls the testes upward, possibly outside the scrotal sac. The patient should be supine and the scrotum elevated by placing a towel under the scrotal sac. A second towel may be used to lay the penis on the anterior aspect of the lower abdominal wall. High-frequency linear transducers in the range of 8–15 MHz with color Doppler capability are necessary. For color Doppler studies, the highest possible power output, lowest scale and wall filter, and optimal gain should be used. The presumed normal testis should be imaged first to optimize scanning parameters. Then, without changing the parameters, the contralateral side should be imaged. This applies to both grayscale and color Doppler techniques. Side-by-side grayscale and color images of both testes should be obtained for comparison. For evaluation of varicocele or inguinal hernia, the patient is also scanned during a Valsalva maneuver and/or while standing. If a tumor of the testis is found and no previous abdominal imaging has been performed, the periaortic and renal hilar regions should be searched for possible lymphadenopathy [4].
MR Imaging Technique
MR imaging of the scrotum is performed with the patient in the supine position. The scrotum should be elevated either with a foam block or towels placed between the thighs. A 3 or 5 in. coil is placed on the area of interest so thin section, high-resolution, small field-of-view images can be obtained. Additionally, depending on the clinical question, a body coil can be used to acquire larger field-of-view images of the pelvis and/or abdomen (generally to the level of the renal hilum). Three orthogonal planes of T2-weighted images and axial T1-weighted images should always be obtained [6, 7]. One plane of the T2-weighted images should have fat suppression to increase dynamic range, increasing sensitivity for subtle differences in signal intensity [6]. If gadolinium is being administered, 2D or 3D fat-suppressed T1-weighted gradient-recalled echo images should be obtained in one plane precontrast and in at least two planes post contrast. As in other body imaging applications, the precontrast fat-suppressed T1-weighted gradient-recalled echo images are the most sensitive for detecting hemorrhage secondary to the increased dynamic range [6].
Normal Ultrasound Appearance of the Scrotum and Contents
The echogenicity of the testis is of intermediate level, which is often compared to that of the thyroid gland and is thought to result from the presence of seminiferous tubules [4]. The mediastinum testis is demonstrated on ultrasound images as a linear echogenic structure [8] (Fig. 26.3). The testis is surrounded by a fibrous capsule, the tunica albuginea, which cannot be seen on ultrasound. Along the length of the testis, the epididymis lies at the posterolateral aspect. Usually only the head of the epididymis is visualized in the normal adult on ultrasound. The echogenicity of the epididymis is similar, or minimally increased, with respect to the testis (Fig. 26.4). Its blood flow is slightly less than that of the testis. Two embryologic remnants measuring a few millimeters each are occasionally visualized when the testis is surrounded by fluid, one at the upper pole of the testis and the other at the head of the epididymis. These are called appendix testis and appendix epididymidis, respectively [4, 9] (Fig. 26.5). The spermatic cord extends superiorly from the testis through the inguinal canal. Pulsed Doppler tracing can recognize the internal spermatic artery by its low-resistance pattern, as opposed to the high-resistance pattern found in the cremasteric and deferential arteries. The spermatic cord contains the vas deferens, a single tubular structure connecting with the seminal vesicles at the base of the bladder to form the ejaculatory ducts that carry the sperm to the urethra. The vas deferens connects with the epididymis at the tail [4].
Key Points
The testes are of intermediate echogenicity (similar to the thyroid gland).
The mediastinum testis is a linear echogenic structure.
The echogenicity of the epididymides is similar to or slightly increased when compared to the testes.
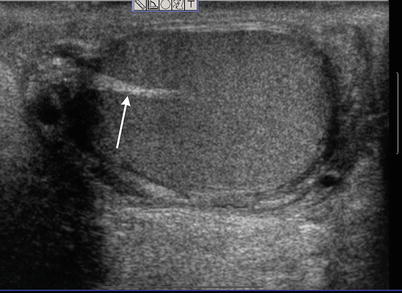
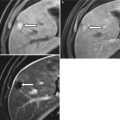
Fig. 26.3
Sagittal ultrasound image of the testis depicting the normal linear echogenic appearance of the mediastinum testis (arrow)
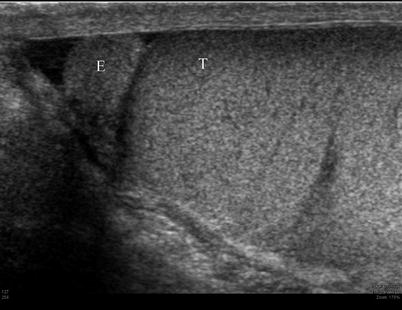
Fig. 26.4
Sagittal ultrasound image of the epididymis and testis demonstrating the similar echogenicity of the epididymis (E) and testis (T)
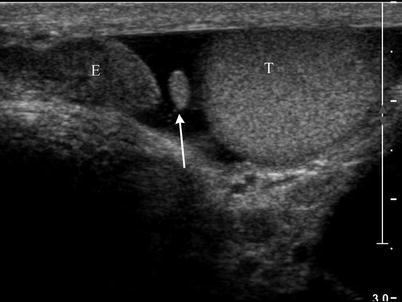
Fig. 26.5
Ultrasound image at the superior pole of the testis demonstrating the normal appearance of the appendix testis (arrow) between the epididymis (E) and testis (T)
Normal MR Imaging Appearance of the Scrotum and Contents
The scrotal wall usually appears as a low T2-weighted signal intensity structure [5, 10]. Normal testes are ovoid structures that show hyperintense T2-weighted signal and isointense T1-weighted signal when compared to skeletal muscle (Fig. 26.6). T2-weighted images provide excellent tissue contrast between the testes and other scrotal structures and are therefore the pillar of scrotal MR imaging [5–7, 10–12]. As in other areas of body imaging, T1-weighted images are useful for tissue characterization, particularly in the depiction of hemorrhage or fat [6, 10, 11]. The testes demonstrate strong, homogenous contrast enhancement following the administration of intravenous gadolinium [5, 10]. The mediastinum testis appears as a hypointense band on T2-weighted images relative to the testicular parenchyma [6, 7, 10, 12] (Fig. 26.6). The tunica albuginea, which is approximately 1 mm thick, shows low T1-weighted and T2-weighted signal intensity, secondary to its fibrous nature [5–7, 10, 11]. The epididymis has similar T1-weighted signal intensity and lower T2-weighted signal intensity than the testis [5–7, 10, 11]. The vas deferens has lowT2-weighted signal intensity in the wall and high T2-weighted signal intensity within the lumen [6].
Key Points
The testes demonstrate increased T2-weighted signal and isointense T1-weighted signal when compared to skeletal muscle.
The testes demonstrate avid enhancement following gadolinium administration.
The mediastinum testis is a hypointense band on T2-weighted images.
The T1-weighted imaging is critical in the depiction of hemorrhage.
The epididymides have similar T1-weighted and decreased T2-weighted signal intensity compared to the testes.
The scrotal wall and testicular capsule demonstrate decreased T2-weighted signal intensity.
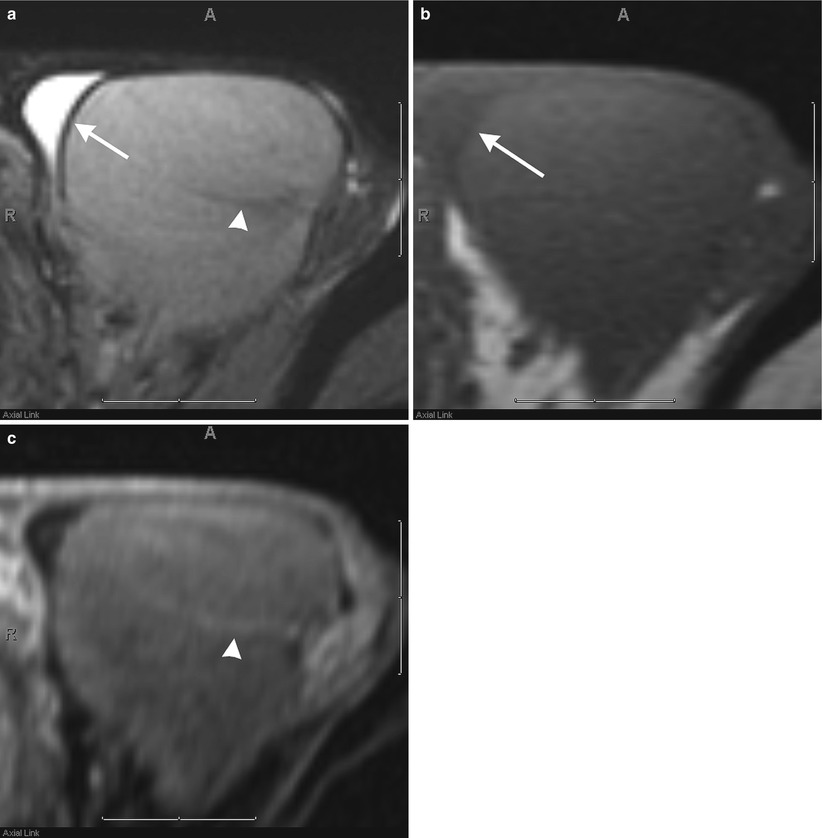
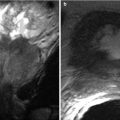
Fig. 26.6
Axial T2-weighted (a), T1-weighted (b), and post-contrast fat-suppressed T1-weighted (c) images of the testis demonstrating the normal appearance of the testicular parenchyma, tunica albuginea (arrow), and mediastinum testis (arrowhead)
Classification of Scrotal Pathologies
Scrotal pathologies can be divided into the following classifications (Table 26.1).
Table 26.1
Classification of scrotal pathologies
Congenital and developmental | Inflammatory/ infectious | Vascular/ perfusion | Trauma | Benign neoplasm | Malignant neoplasm |
|---|---|---|---|---|---|
Supernumerary testes | Sarcoidosis | Testicular torsion | Hematocele | Testicular cyst | Seminomatous/ nonseminomatous germ cell tumors |
Undescended testes | Tuberculosis | Testicular infarction | Intratesticular hemorrhage | Epididymal cyst/spermatocele | Lymphoma |
Dilated rete testis | Epididymitis/ epididymo-orchitis | Varicocele | Testicular rupture/fracture | Tunical cyst | Pleomorphic undifferentiated sarcoma |
Inguinal hernia | Pyocele | Intratesticular varicocele | Prosthesis | Epidermoid inclusion cyst | Metastases |
Splenogonadal fusion | Fournier’s gangrene | Testicular adrenal rest tumor (TART) | Rhabdomyosarcoma | ||
Sperm granuloma | Leydig cell hyperplasia | Liposarcoma | |||
Sclerosing lipogranuloma | Leiomyoma | Malignant Schwannoma | |||
Fibrous pseudotumor | Leiomyosarcoma | ||||
Lipoma (including spermatic cord) |
Algorithmic Approach to Scrotal Masses
When approaching scrotal masses, the two most important considerations are whether the lesion is intratesticular or extratesticular and whether the lesion is solid or cystic (Table 26.2).
Table 26.2
Scrotal mass
Intratesticular Likely malignant | Extratesticular Likely benign | ||
|---|---|---|---|
Solid | Cystic | Solid | Cystic |
Primary neoplasm | Testicular cyst | Inguinal hernia | Hydrocele |
Lymphoma | Dilated rete testis | Spermatic cord lipoma | Hematocele |
Metastases | Adenomatoid tumor | Pyocele | |
Leydig cell hyperplasia | Sperm granuloma | Fournier’s gangrene | |
Granulomatous disease | Leiomyoma | Tunical cyst | |
Epidermoid | Papillary cystadenoma | Epididymal cyst | |
Adrenal rests | Fibrous pseudotumor | Spermatocele | |
Prosthesis | Sarcoma | Varicocele | |
Calculi | |||
Splenogonadal fusion | |||
Sclerosing lipogranuloma | |||
Cryptorchidism | |||
Polyorchidism | |||
Intratesticular Lesions
While the dichotomous division of intratesticular scrotal masses into solid and cystic lesions is useful for the purposes of developing a differential diagnosis, it may also be helpful to approach intratesticular masses as single and unilateral or multiple and bilateral (Table 26.3).
Table 26.3
Intratesticular masses
Single unilateral | Multiple bilateral | |
|---|---|---|
Focal | Diffuse | |
Seminoma | Nonseminomatous germ cell tumor | Lymphoma |
Epidermoid | Dilated rete testis | Metastases |
Prosthesis | Orchitis | Leydig cell hyperplasia |
Testicular cyst | Granulomatous disease | |
Adrenal rests | ||
Solid
Solid intratesticular lesions, unlike extratesticular lesions, are highly suspicious for malignancy [6, 10]. Most intratesticular neoplasms are isointense to the testis on T1-weighted images and hypointense to the normal testis on T2-weighted images [5–7, 13, 14]. Testicular malignancies demonstrate more heterogeneous enhancement than normal testicular parenchyma [6, 10, 13, 15] (Table 26.4). Ultrasonographic appearance and MR findings in conjunction with serum markers (lactate dehydrogenase, alpha fetoprotein, and beta-human chorionic gonadotropin) help to distinguish primary germ cell tumors from secondary malignancies of the testis; however, the distinction between primary and secondary tumors and benign and malignant tumors cannot be made on imaging findings alone [6, 7, 10, 12–15]. Primary testicular tumors are staged using TNM staging, which include serum tumor marker evaluation (Tables 26.4 and 26.5).
Table 26.4
MR imaging features of solid intratesticular lesions
Malignant | T1 SIa | T2 SIa | Enhancementa |
|---|---|---|---|
Seminomatous germ cell neoplasms | Isointense | Decreased | Homogeneous increased |
Nonseminomatous germ cell neoplasms | Heterogeneous (due to fat hemorrhage or calcium) | Heterogeneous | Heterogeneous increased |
Lymphoma | Heterogeneous | Heterogeneous | Heterogeneous |
Metastases | Variable | Variable | Variable |
Benign | |||
Leydig cell hyperplasia | Isointense | Decreased | Mild |
Granulomatous disease | Isointense | Decreased | Decreased |
Epidermoid inclusion cyst | Isointense | Decreased with alternating bands | None |
Testicular adrenal rests | Iso to slightly hyperintense | Decreased | Diffusely increased |
Prostheses | Decreased | Decreased | None |
Table 26.5
Staging of testicular cancer (Adapted from the NCI Testicular Cancer Treatment PDQ®)
Stage 0 | Carcinoma in situ (intratubular tumor) |
Stage IA | 1. Tumor involving the testis and epididymis without spread beyond the visceral layer of the tunica vaginalis |
2. Normal serum tumor markers | |
Stage IB | 1. Tumor involving the testis and epididymis with spread beyond the visceral layer of the tunica vaginalis (blood vessels, lymphatics, spermatic cord, or scrotum) |
2. Normal serum tumor markers | |
Stage IS | 1. Tumor anywhere in the testis, spermatic cord, or scrotum |
2. All tumor markers are elevated or one tumor marker is moderately above normal or high | |
Stage II | 1. Tumor anywhere in the testis, spermatic cord, or scrotum with metastases to subdiaphragmatic lymph nodes |
2. Tumor markers are normal or slightly elevated | |
Stage IIA | Metastasis in up to 5 lymph nodes, none larger than 2 cm |
Stage IIB | 1. Metastasis in up to 5 lymph nodes, at least one >2 cm, all ≤5 cm |
2. Metastasis to more than 5 lymph nodes, ≤5 cm | |
Stage IIC | Lymph nodes >5 cm |
Stage III | Tumor anywhere in the testis, spermatic cord, or scrotum with metastases to lymph nodes above the diaphragm or the lung |
Stage IIIA | Tumor markers are normal to slightly elevated |
Stage IIIB | One or more tumor markers are moderately above normal |
Stage IIIC | 1. One or more tumor markers are high |
2. There are other distant metastases |
Key Points
Solid intratesticular lesions are highly suspicious for malignancy.
Most intratesticular neoplasms demonstrate similar T1-weighted and increased T2-weighted signal intensity when compared to the normal testis.
Testicular malignancies demonstrate more heterogeneous enhancement than normal testicular parenchyma.
Primary Testicular Malignancies
Primary testicular neoplasms are of germ cell origin 95 % of the time. These germ cell neoplasms are evenly split between seminomatous and nonseminomatous germ cell tumors. Distinction between seminomatous and nonseminomatous germ cell tumors is important for determining treatment and prognosis [13]. Seminoma is the most common cell type and tends to appear more homogenous on sonography and all MR pulse sequences, where as nonseminomatous germ cell tumors tend to be more heterogeneous secondary to hemorrhage and mixed cell type [6, 10, 12, 13]. Seminomas are homogeneously hypoechoic on ultrasound, making them easy to distinguish from the normal testicular parenchyma [8, 9]. Seminomas are multinodular tumors of uniform signal intensity that are hypointense on T2-weighted images [6, 7, 10–14] (Fig. 26.7). The internal fibrovascular septa within seminomas may appear as band-like areas of decreased T2-weighted signal intensity which demonstrate greater enhancement following intravenous gadolinium administration than the remainder of the tumor tissue [10, 13]. Nonseminomatous tumors tend to be large and, because of their mixed cell type, may have internal hemorrhage, necrosis, or calcification [6, 7, 10–14]. The heterogeneous makeup leads to heterogeneous echogenicity on ultrasound, sometimes with acoustic shadowing related to calcifications [8, 9]. Similarly, on MR imaging, nonseminomatous germ cell tumors tend to be larger and heterogeneous in signal intensity on all pulse sequences [6, 7, 10–14] (Fig. 26.8).
Key Points
95 % of primary testicular neoplasms are of germ cell origin.
Germ cell neoplasms are split 50/50 between seminomatous and nonseminomatous.
Seminomas are homogeneous on ultrasound and all MR pulse sequences.
Nonseminomatous germ cell neoplasms are heterogeneous on ultrasound and all MR pulse sequences.
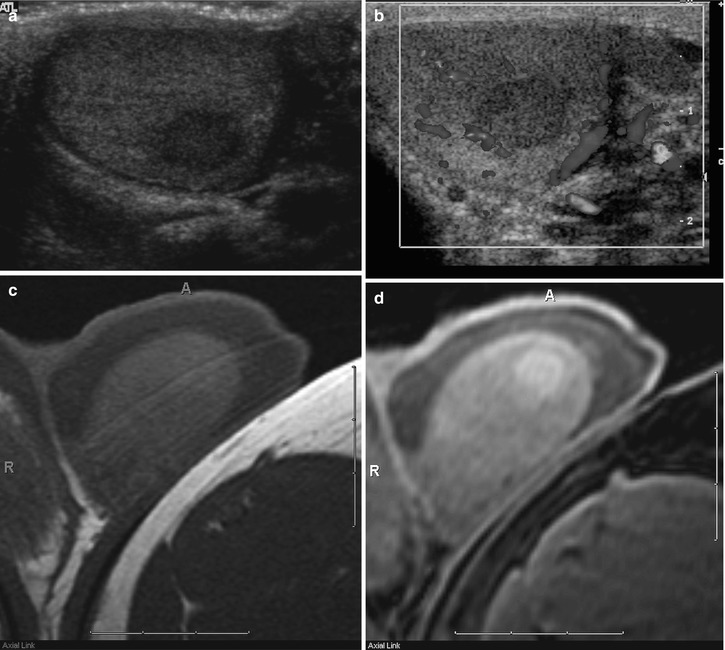
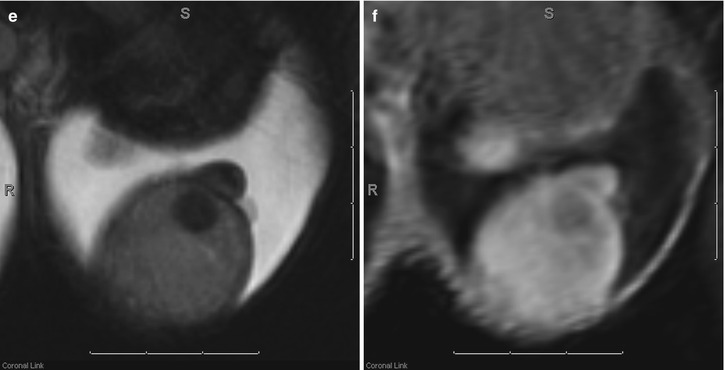
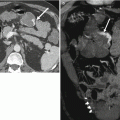
Fig. 26.7
Grayscale (a) and color Doppler (b) images of a left testicular seminoma demonstrating characteristic decreased echogenicity and decreased color flow in comparison with the normal testicular parenchyma. Axial T1-weighted (c) and fat-suppressed T1-weighted (d) MR images demonstrate that the seminoma is nearly isointense to the normal testicular parenchyma. Only after increasing the dynamic range by adding fat suppression can the subtle T1-weighted hyperintensity of the seminoma be appreciated. Coronal T2-weighted (e) and coronal post-contrast fat-suppressed T1-weighted (f) MR images demonstrate the characteristic decreased T2-weighted signal intensity and decreased contrast enhancement of the seminoma when compared with the normal testicular parenchyma
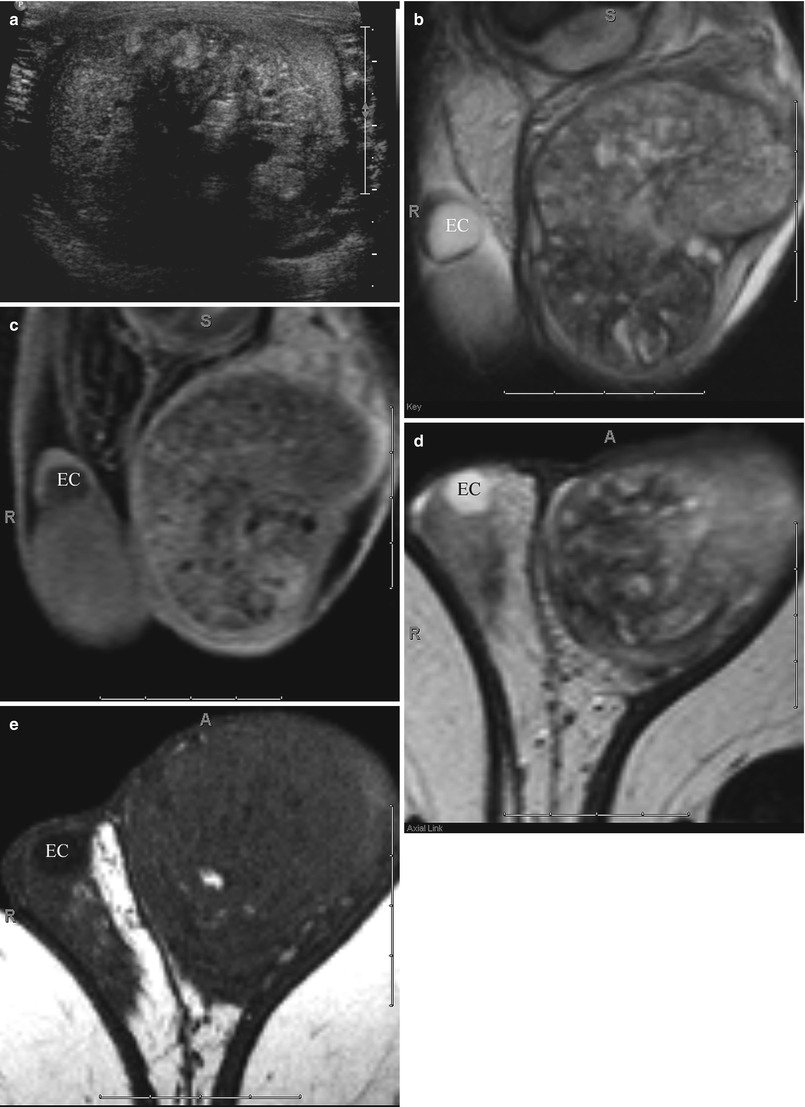
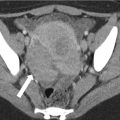
Fig. 26.8
Ultrasound (a), coronal T2-weighted (b), and post-contrast T1-weighted (c), as well as axial T2-weighted (d) and T1-weighted (e) MR images demonstrating the more heterogeneous appearance of a mixed germ cell tumor. Also noted is aright epididymal head cyst (EC)
Lymphoma
Non-Hodgkin’s lymphoma is the most common intratesticular neoplasm in men over the age of 50 and may either be primary or systemic. Testicular lymphoma is more often multifocal and infiltrative as compared to primary testicular malignancies. Testicular recurrence of lymphoma is common due the relative impermeability of the blood-testis barrier to chemotherapeutic agents [5–7, 10]. The sonographic appearance is generally homogenous, hypoechoic testes or multifocal, hypoechoic lesions of varying sizes. Striated hypoechoic bands radiating from the mediastinum testis (also known as striated testis) have also been described. These lesions demonstrate increased color flow on Doppler evaluation regardless of lesion size [8–10] (Fig. 26.9). MR imaging appearance is similar to that of nonseminomatous germ cell tumors but is not reliable for distinguishing these entities apart from one another [6].
Key Points
Lymphoma is the most common intratesticular neoplasm in men over 50.
Most often multifocal and infiltrative.
Testicular recurrence is common due to the relative impermeability of the blood-testis barrier to chemotherapeutic agents.
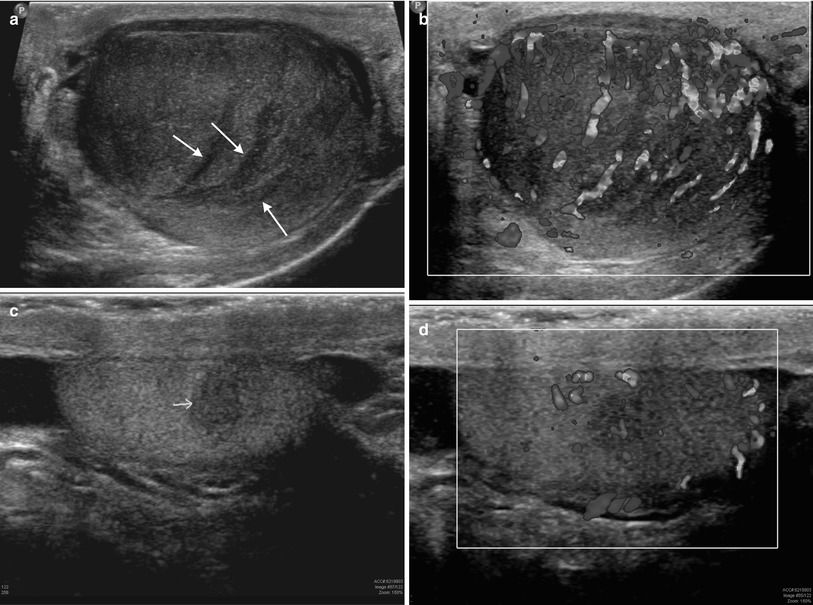
Fig. 26.9
Grayscale and color Doppler images of the right and left testes (a–d, respectively) in a 67-year-old male with a history of non-Hodgkin’s lymphoma demonstrating multifocal hypoechoic lesions of varying sizes with increased color flow. Also note the “striated testis” appearance (arrows) of the right testis (a)
Metastases
Metastases to the testicle are rare, but have been reported in cases of melanoma, prostate carcinoma, and lung carcinoma. Metastases to the testes are generally seen in widespread metastatic disease and are rarely the primary complaint [7, 10]. History is critical in diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree