The Superficial Soft Tissues
Lee F. Rogers
Carol A. Boles
Pamela A. Propeck
L. F. Rogers and C. A. Boles: Department of Radiology, Wake Forest University School of Medicine, Winston-Salem, North Carolina 27157. P. A. Propeck: University of Wisconsin Hospital and Clinics, Madison, Wisconsin 53792-3252.
GENERAL CONSIDERATIONS
Lee F. Rogers
Carol A. Boles
The soft tissues can be seen in every roentgenogram, even those that have been heavily exposed, by viewing the film with a strong beam of light (in addition to the usual illuminators). Some type of spotlight should always be available for the scrutiny of overexposed roentgenograms and particularly for the study of the soft tissues.
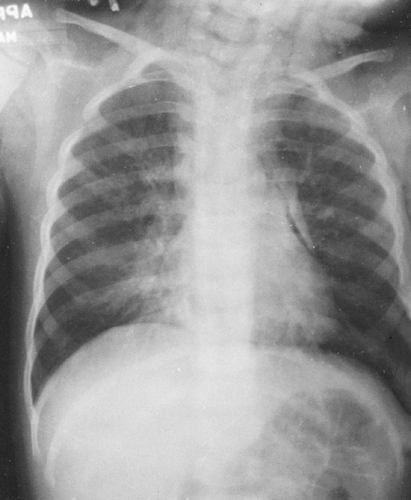
Although the density of the soft tissues as a whole is close to that of water, there is enough difference between fat and other tissues to make subcutaneous fat distinctly visible on roentgenograms as a more translucent area beneath the skin (Fig. 10-1). The fat also makes the outer surface of the muscles stand out clearly. Localized accumulations of fat near the joints aid in the recognition of joint effusion because of their displacement when the joint capsule is distended. If there has been wasting from disease with consequent loss of fat, the soft parts have a very homogeneous density.
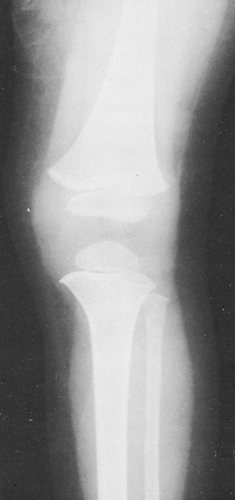 FIG. 10-1. Normal soft tissues. This film demonstrates the difference in density of muscle and fat. The more radiolucent subcutaneous fat causes the muscles to stand out clearly. |
When specifically indicated, a suitable selection of exposure factors (26 to 40 kVp) with relatively low filtration (e.g., 0.5 mm of aluminum) produces a roentgenogram of high quality in which the soft-tissue outlines are preserved and good differentiation of the soft-tissue densities is obtained. This type of roentgenogram is useful when the examination is made primarily for soft-tissue evaluation. These factors have been used in mammography.
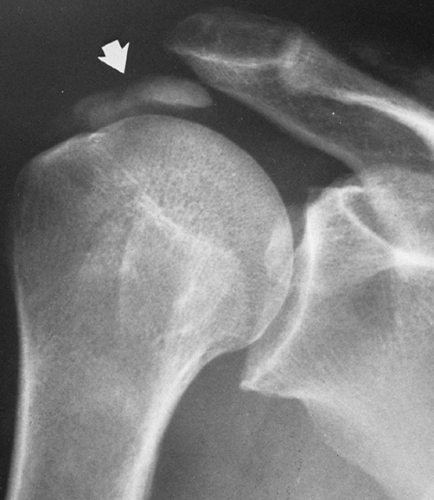
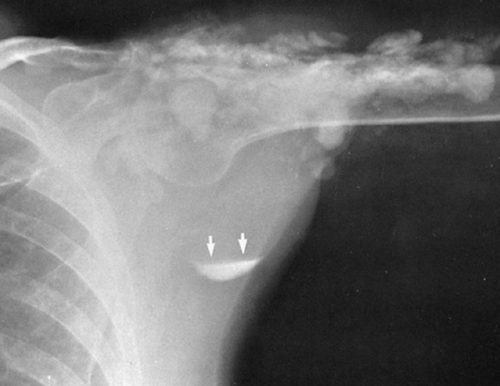
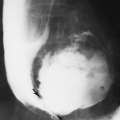
A small, localized soft-tissue enlargement such as a wart or a mole in close contact with the film cassette or tabletop can cause a sharply marginated increase in density that may be mistaken for a pathologic process within the tissues. It can be seen because it contrasts with the surrounding air. If a similar mass is placed within the abdomen, it cannot be visualized separately from the surrounding soft tissues of similar density. For example, a mole on the surface of the chest wall may produce a round density that can be mistaken for a nodule within the lung, and a similar lesion of the soft tissues of the lower back may suggest a renal or gallbladder calculus (Fig. 10-2). The nipples are a common source of difficulty in the interpretation of chest roentgenograms. If one breast is pressed more firmly against the film cassette than the other, the nipple on one side may form a sharply outlined round density while the other is invisible. Absence of one breast after radical mastectomy causes one lung to be more translucent than the other.
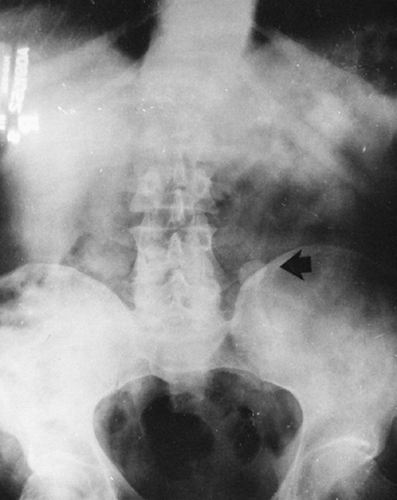 FIG. 10-2. A mole on the skin of the back was responsible for the round density (arrow) seen in this film of the abdomen. |
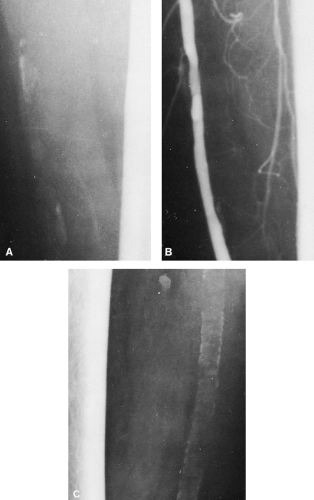
Contrast between soft tissues can be achieved by the injection of contrast material (i.e., angiography), which allows visualization of the lumina of arteries of the solid organs by perfusion (see Fig. 10-12). Computed tomography (CT) demonstrates the soft tissues with greater clarity than plain film radiography by clearly depicting the fat in fascial planes surrounding muscles and vascular structures (Fig. 10-3); however, the density of these structures is similar, requiring the use of contrast agents for the identification of vascular structures. Magnetic resonance imaging (MRI) distinguishes among various soft-tissue structures by their different signal intensities (see Fig. 10-29). These can be varied by changing the parameters of the examination to exploit or modify the relative signal intensities from differing tissues. Vascular structures are directly identified by signal voids or variations created by the flow of blood. The use of sonography in the assessment of soft tissues continues to increase beyond its initial task of differentiating between cystic and solid structures. Ultrasound can be used to evaluate tissue interfaces and to assess tendon injuries. These newer technologies have freed the soft tissues from the radiographic shadows.
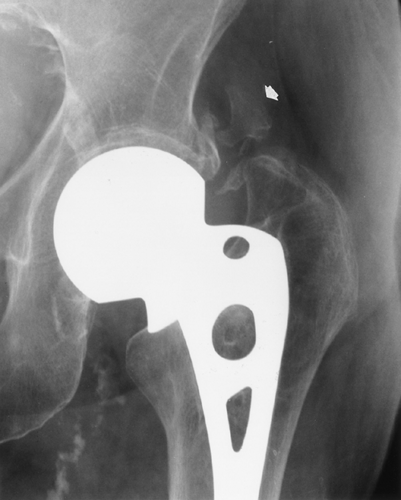 FIG. 10-5. Heterotopic ossification after hip arthroplasty. The solid bone adjacent to left unipolar hip arthroplasty (arrow) was not present immediately after surgery. |
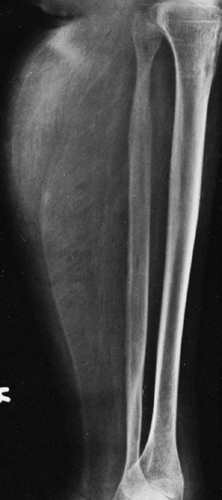 FIG. 10-8. Pseudohypertrophic muscular dystrophy. The muscle of the calf is very large but contains fat, producing streaks of radiolucency. Note the wide fibular shaft. |
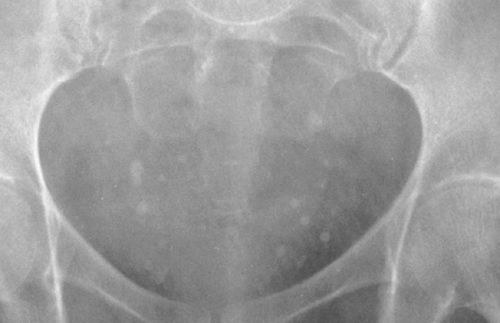 FIG. 10-13. Phleboliths in pelvis. Numerous rounded, calcific densities in the pelvis are typical of phleboliths. Note that many are slightly more dense on their periphery than centrally. |
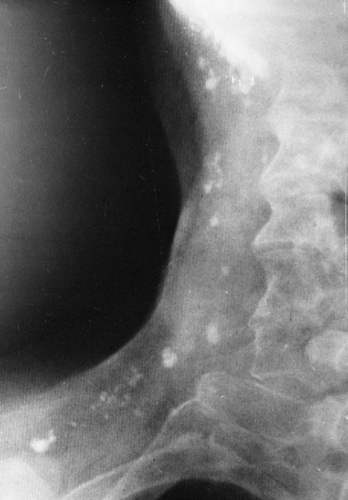 FIG. 10-16. Calcified cervical lymph nodes. Multiple calcifications showing a variety of sizes and shapes are observed in the cervical nodes. |
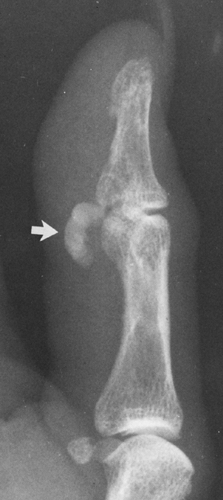 FIG. 10-19. Calcific tendinitis involving the flexor tendons of the thumb. Note the crescentic, homogeneous calcification (arrow). The smaller, underlying ossific densities represent sesamoid bones. |
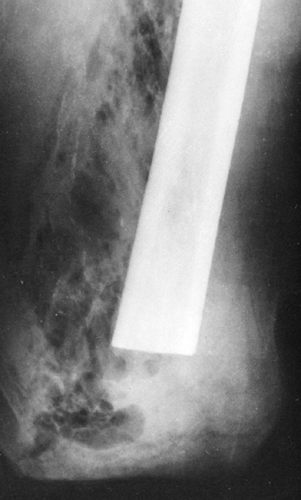 FIG. 10-28. Gas gangrene of the thigh after amputation. The gas forms irregular streaks and rounded translucent areas in the soft tissue of the thigh. |
DISORDERS OF MUSCLE
Myositis Ossificans and Heterotopic Bone Formation
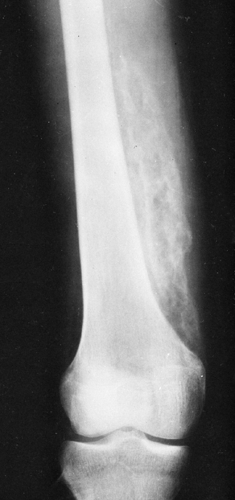
Calcification with subsequent ossification often follows trauma to the deep tissues of the extremities and is called traumatic myositis ossificans. In about one third of cases, a history of trauma is absent. Myositis ossificans may follow any local injury sufficient to cause bruising of the muscle or a frank hemorrhage within it. The rectus femoris and the brachialis are the two most frequently affected muscles in the body. Involvement of muscles of the thigh is observed frequently in athletes, particularly football players. An injury that is severe enough to cause a deep muscle bruise often traumatizes the periosteum as well, which may be elevated by hemorrhage. This calcifies as it matures over time.
Trauma leads to a proliferative repair within the connective tissue without frank inflammation. Osteoprogenitor cells, which are the source of heterotopic bone, are induced during this time of repair. Clinically, there is localized redness, heat, pain, and loss of function. The first finding is that of soft-tissue swelling. Peripheral calcification may become visible as a hazy shadow of increased density within a few weeks after the initiating trauma. Calcification subsequently develops in a centrifugal pattern, which is flocculent at first. Over a period of several weeks, it gradually becomes more dense and finally develops the appearance of actual bone. The mass has a laminated, or zonal, character and is characteristically more densely calcified on its periphery than centrally (Fig. 10-4). The exact appearance of the lesion is closely related to its age and parallels the stage of maturation. Usually, after a period of about 6 months, the ossification gradually decreases in size; smaller masses may disappear completely.
The active nature of the process results in the uptake of radioactive bone-seeking agents. In general, plain films are sufficient for diagnosis, especially if there is a history of trauma. CT scanning may be useful to demonstrate the peripheral
calcification if the lesions are atypical on conventional radiographs.64,79
calcification if the lesions are atypical on conventional radiographs.64,79
Surgery
Heterotopic new bone may form after surgery about the joints, particularly after total hip arthroplasty. This new bone may be poorly defined initially, but it gradually matures to the characteristic appearance of bone (Fig. 10-5).
Burns
Heterotopic soft-tissue calcifications occur in about one fourth of patients with severe burns. They tend to be flocculent, and they occur more commonly in children than in adults. Some spontaneous regression is the rule unless tissues adjacent to and surrounding a joint are involved. In that case, a bony bridge may form, resulting in immobilization of the joint.
Spinal Cord Injury
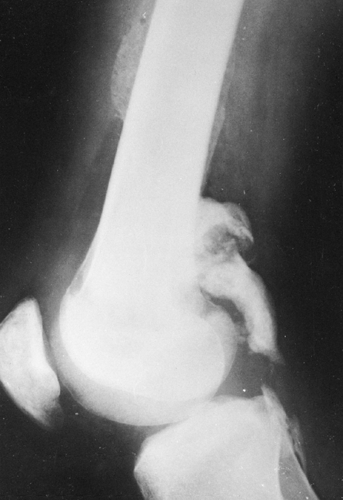
Muscle ossification is also found in 30% of patients after spinal cord injury or other central nervous system disease that results in paralysis. Heterotopic bone frequently forms distal to the level of cord injury and may lead to fusion across joints. Heterotopic ossification most frequently occurs about the hip, although it can occur about any joint (Fig. 10-6). There is usually no history of trauma or inflammation in the affected region. The process begins as early as 3 to 4 weeks after injury and continues actively for 2 to 3 years to form mature bone. Early in the process the calcification is filamentous. When it is mature, radiographs demonstrate irregular sheets, bands, and plaques of bone. Surgery is occasionally required to excise a segment of the ossification to free a completely immobilized joint. Ossification may recur if surgery is performed while the process is active; skeletal scintigraphy may be performed before contemplated surgery to assess the activity of the process.
Head Injury
Patients who are comatose from head injury may experience heterotopic ossification similar to that seen in patients with an injured spinal cord.
Myositis Ossificans Progressiva
Myositis ossificans progressiva is a rare disorder of unknown cause. It appears to be a congenital dysplasia. It begins in early childhood with the development of doughy and often painful swellings, chiefly in the muscles of the neck and back. As these swellings subside, a diffuse fibrosis is left, and this in turn is followed by the development of plate-like masses of bone (Fig. 10-7). There is a slow progression. The abnormal ossifications are often observed first in the muscles of the neck and back. Initially, these ossifications are relatively hazy and somewhat difficult to identify. In
time, irregular, elongated bony plates are observed extending along the long axis of the involved muscle. In later stages, numerous muscles become extensively ossified and the joints become virtually immobile. Associated anomalies of the small bones of the hands and feet are found in almost all patients.
time, irregular, elongated bony plates are observed extending along the long axis of the involved muscle. In later stages, numerous muscles become extensively ossified and the joints become virtually immobile. Associated anomalies of the small bones of the hands and feet are found in almost all patients.
Muscular Dystrophies
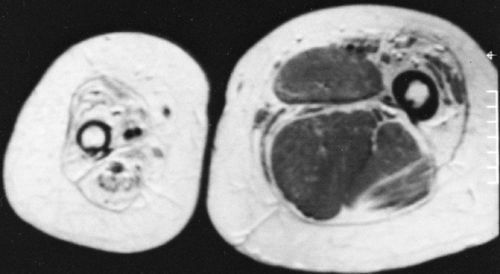
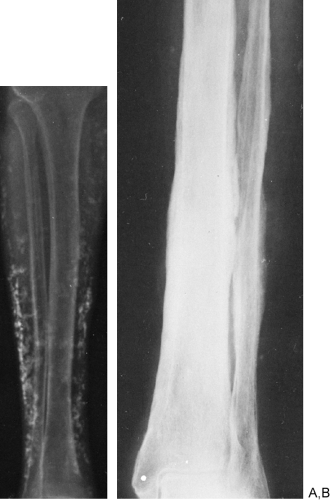
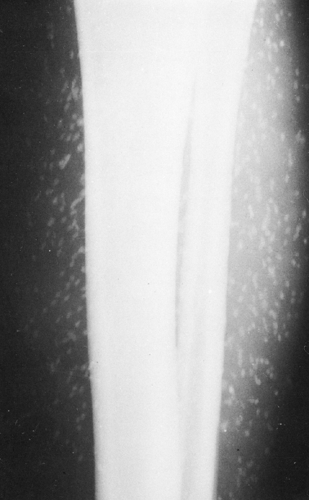
The replacement of muscle by fat in muscular dystrophies results in a fairly characteristic appearance on roentgenograms of the extremities. The muscles do not shrink appreciably in size, but the extensive accumulation of fat within the remaining muscle bundles gives them a finely striated or striped appearance. In later stages, most of the muscle tissue is replaced by fat, and the fascial sheath bounding the muscle stands out as a thin line of increased density because it is visualized on edge (Fig. 10-8). One of the clinical subgroups is known as pseudohypertrophic muscular dystrophy (Duchenne’s syndrome). It is characterized by the enlargement of certain muscle groups, usually those of the calves and shoulder girdles. The appearance clinically is that of a very muscular individual, but strength actually is markedly decreased. In addition to the extensive replacement by fat in this type of dystrophy, the muscles are enlarged. This is the only condition in which there is the combination of large muscles interlaced with fat (Fig. 10-8).
Muscle Atrophy
In patients with long-standing paralysis of one or more extremities—including such conditions as poliomyelitis (see Fig. 10-3), spinal cord injuries, and cerebral vascular accidents—muscles may contain stripes of fat. The affected muscle groups are decreased in size, but the subcutaneous fat layer may be thick. If complete paralysis of an extremity has existed for a long time, the muscle bundles are almost completely absent, and subcutaneous fat makes up most of the soft tissues that surround the bones.
Muscle Injury
Evaluation of muscle injury after trauma is often straightforward, and conservative treatment methods obviate the need for imaging. Often, however, the extent of injury cannot be determined by physical examination because groups of
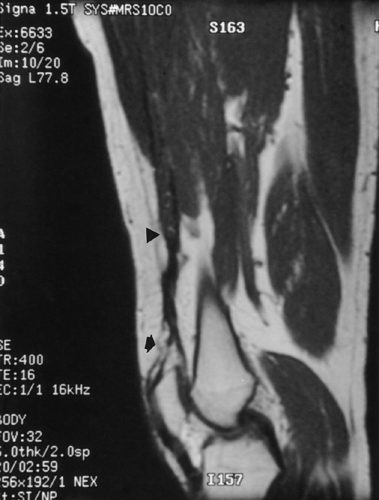
muscles act in concert, with compensation by the other muscles within the group when one is injured. High-performance athletes may require imaging to better delineate the site and extent of injury and aid in treatment and prognosis. Imaging may also be useful when the physical examination is equivocal. MRI is the most useful method to evaluate the extent of hemorrhage and edema within a muscle or group of muscles (Fig. 10-9). Partial and complete tendon avulsions can also be diagnosed (Fig. 10-10). The edema and hemorrhage within and around the injured muscle demonstrates increased signal on T2-weighted images.64 MRI is most frequently used to evaluate soft-tissue injuries about the joints (see Chapter 2).
muscles act in concert, with compensation by the other muscles within the group when one is injured. High-performance athletes may require imaging to better delineate the site and extent of injury and aid in treatment and prognosis. Imaging may also be useful when the physical examination is equivocal. MRI is the most useful method to evaluate the extent of hemorrhage and edema within a muscle or group of muscles (Fig. 10-9). Partial and complete tendon avulsions can also be diagnosed (Fig. 10-10). The edema and hemorrhage within and around the injured muscle demonstrates increased signal on T2-weighted images.64 MRI is most frequently used to evaluate soft-tissue injuries about the joints (see Chapter 2).
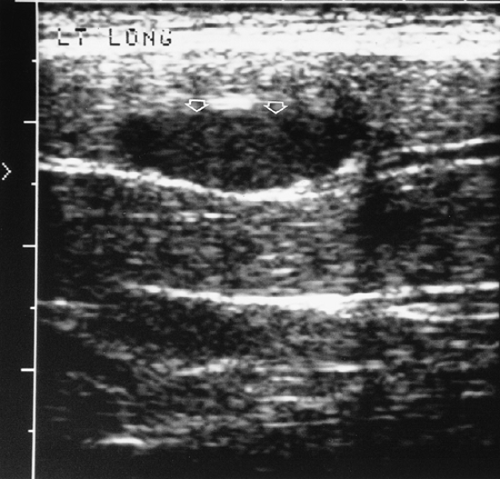
Ultrasound has an increasing role in the evaluation of soft-tissue injuries. A tendon tear or rupture may be diagnosed by demonstration of cystic areas within the normally uniformly echogenic tendon. This is most often used to evaluate the tendons of the rotator cuff and the Achilles tendon. Vascular malformations can be evaluated by demonstration of characteristic flow. Masses about joints can be confirmed as ganglia or popliteal cysts when they are anechoic, well defined, and have good through transmission. Hematomas typically are relatively anechoic with variable septations (Fig. 10-11).31
CALCIFICATION IN THE SOFT TISSUES
Calcification in the soft tissues can arise from a number of causes. It can be dystrophic, occurring in necrotic tissues, as in infections or tumors; it can be metabolic, occurring as the result of deposition due to abnormal concentrations of calcium salts, as in chronic renal failure or calcium deposition disease; or, it can represent ossification, which occurs in certain chondral tumors of soft tissues.
Arterial Calcification
Calcification in the walls of the larger arteries of the abdomen and of the extremities is a frequent observation on roentgenograms of persons of middle age or older. Intimal arteriosclerosis is characterized pathologically by the formation of atheromatous plaques in the thickened intima of the arteries. The atheromatous plaques may or may not be calcified. When they are calcified, they are seen as irregular plaques of variable size, from small flecks to larger areas 1 cm or more in length. They may be elongated or somewhat triangular, with a considerable variation in shape. They seldom completely encircle the lumen of the vessel and are distributed irregularly along the vessel’s course (Fig. 10-12). The amount of visible calcification bears no relation to the severity of the vascular occlusion; complete obstruction can exist with no visible calcification.
Mönckeberg’s medial arteriosclerosis is characterized by the deposition of calcium in the media of the vessel. These deposits do not narrow the vessel lumen, nor do they interfere with flow. Mönckeberg’s arteriosclerosis is an almost constant finding in elderly persons, and it is frequently seen in those between 35 and 50 years old, particularly in diabetics. The vessels most often affected are the femoral, popliteal, and radial arteries. The calcification occurs in the form of closely spaced, fine, concentric rings. These may be complete or incomplete, but the process generally is diffuse, involving long segments of multiple vessels (see Fig. 10-12C).
Calcification of Veins
Phleboliths
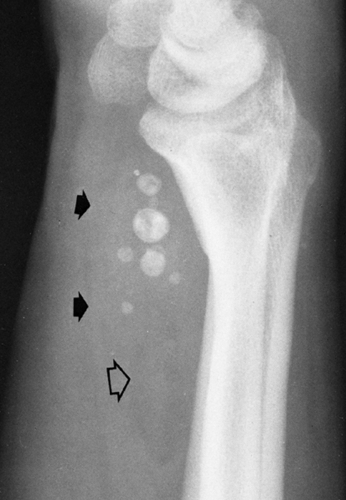
A phlebolith is a calcified thrombus within a vein. Phleboliths are found very frequently in the pelvic veins, and most adults have a few of them. They occur in the form of small, round or slightly ovoid, calcified shadows of variable size, from very tiny ones up to about .5 cm in diameter (Fig. 10-13). They may be of homogeneous density, be laminated, or have a ring-like appearance. Phleboliths are common in varicose veins of the lower extremities. They frequently form in the dilated venous spaces of a cavernous
hemangioma and result in one of the characteristic roentgenographic signs of the lesion. When a number of small, rounded calcifications are seen in a localized area of the superficial soft tissues, one should consider the possibility of a cavernous hemangioma (Fig. 10-14). The phleboliths in a hemangioma very often have a ring-like appearance.
hemangioma and result in one of the characteristic roentgenographic signs of the lesion. When a number of small, rounded calcifications are seen in a localized area of the superficial soft tissues, one should consider the possibility of a cavernous hemangioma (Fig. 10-14). The phleboliths in a hemangioma very often have a ring-like appearance.
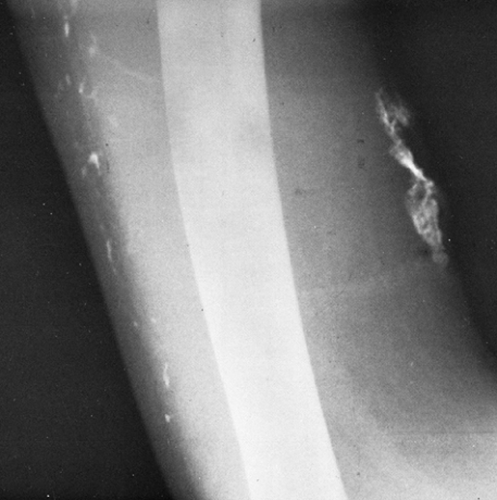
Calcification Associated with Venous Stasis
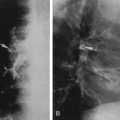
In the presence of venous stasis of long duration, usually secondary to varicosities and thromboses, thin, stripe-like shadows of calcification may be seen in the subcutaneous tissues. These usually appear as double parallel stripes or with distinctly tubular and branching characteristics. Additionally, plaque-like calcifications are often present in the subcutaneous tissues throughout the legs (Fig. 10-15A) or as a more localized process in the neighborhood of a varicose ulcer. The calcification in some of these patients resembles very closely that seen in patients with diffuse scleroderma or dermatomyositis, except that it is localized to the leg. Phleboliths are often seen in association with the plaques. Smooth, wavy periosteal reaction along the tibial and fibular shafts is common (Fig. 10-15B). The stasis ulcers that are often present may be seen as defects in the soft tissues when the film is viewed with a high-intensity light.
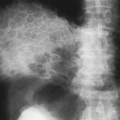
Calcification of Lymph Nodes
After being involved by infection, usually tuberculosis or histoplasmosis, the peripheral lymph nodes may calcify. The most common site is the cervical chain (Fig. 10-16), with the axillary nodes second in frequency. Calcification of other peripheral nodes is very uncommon. The calcifications are visualized as mottled areas of calcific density, often multiple, which are distributed along the course of the cervical lymph node chains or in the axilla.
Parasitic Calcification
Cysticercosis
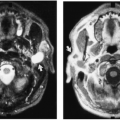
The larvae of the pork tapeworm (Taenia solium), known as Cysticercus cellulosae, may lodge in the brain, meninges, muscles, and other structures and become encysted. Usually the pig is the intermediate host, and human infestation occurs from eating improperly cooked pork. If the eggs are swallowed, the result is self-infection as the larvae enter various tissues and become encysted. Therefore, humans also act as an intermediate host. The encysted parasites may become sufficiently calcified to be visualized roentgenographically as small or slightly elongated masses of one to several millimeters in diameter or length. The small size of the calcifications and their wide dissemination, particularly in the brain, meninges, and muscles, is very suggestive of the diagnosis (Fig. 10-17).
Trichinosis
The encysted embryos of Trichinella spiralis are said to undergo calcification frequently, but the parasite is so small that it cannot be seen readily on roentgenograms. Therefore, the diagnosis of trichinosis usually cannot be made from roentgenographic examination.
Hydatid Disease
Hydatid disease is caused by infestation with hydatid cysts, the larval forms of an echinococcal tapeworm. The cysts are usually found in visceral organs of the thorax and abdomen. Rarely, they occur in the soft tissues of the extremities, where they tend to be small and fragmented, in contrast to large visceral cysts. Therefore, the appearance is not characteristic, and the calcifications may exist in a variety of bizarre patterns.
Dracunculiasis (Guinea Worm Infestation)
Guinea worm infestation may be manifested on roentgenograms when fibrositis or myositis develops around a dead female guinea worm that migrates in the subcutaneous tissues before discharging eggs. The worm may calcify, particularly if it remains deep in the tissues. It appears as a long, string-like calcification, up to 10 to 12 cm, usually in the lower limbs.
Articular and Periarticular Calcification
Calcific Bursitis and Tendinitis
Inflammatory changes in tendons and bursae are common, particularly in the shoulder. This results in pain and limitation of motion, and it is the most frequent cause of shoulder disability. It is referred to variously as bursitis, tendinitis, calcific tendinitis, or, more obscurely, as peritendinitis calcarea. Radiographically, calcium deposits may be identified in the tendons of the rotator cuff. These usually occur in the tendon of the supraspinatus and are found directly above the greater tuberosity of the humerus (Fig. 10-18). Similar
deposits in the tendons of the infraspinatus, subscapularis, and teres minor components of the cuff are less common. These deposits in the tendons are often associated with inflammation of an overlying bursa; hence, the clinical designation of bursitis or subacromial bursitis. The mass of calcium may erupt into the bursa, or the calcium may be spontaneously resorbed.
deposits in the tendons of the infraspinatus, subscapularis, and teres minor components of the cuff are less common. These deposits in the tendons are often associated with inflammation of an overlying bursa; hence, the clinical designation of bursitis or subacromial bursitis. The mass of calcium may erupt into the bursa, or the calcium may be spontaneously resorbed.
It is not at all uncommon to find calcium deposits around the shoulder in patients who have no complaints, or at least none at the time of the examination. Therefore, the mere presence of demonstrable calcium does not indicate the existence of an acute inflammatory process.
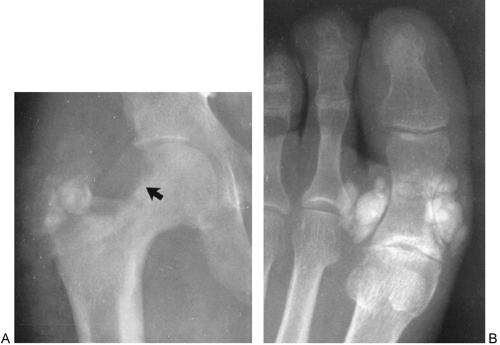
In addition to their presence in the shoulder, calcifications of a similar nature are at times found in the trochanteric bursa that overlies the greater trochanter of the femur. They are also encountered in the periarticular tissues around the elbow, hand (Fig. 10-19), or wrist; around the prepatellar
bursa of the knee; and in the retropharyngeal tissues of the upper cervical spine.37
bursa of the knee; and in the retropharyngeal tissues of the upper cervical spine.37
Calcium Hydroxyapatite Deposition Disease
Calcium hydroxyapatite has been identified as the crystal most commonly responsible for calcium deposits in tendons and bursae. This and other related calcium phosphate crystals may occur in association with both intra-articular and periarticular changes involving one or many joints. The combination of chondrocalcinosis and periarticular soft-tissue calcium deposits is known as mixed calcium phosphate deposition disease.42,70 Calcium hydroxyapatite crystals are so small (75 to 250 nm) they are not individually visible by light microscopy, and they are seen only in the aggregate. Therefore, the diagnosis is usually presumptive and based on the radiographic findings.
Calcium Pyrophosphate Deposition Disease
The principal finding in this disease is chondrocalcinosis—calcium salts deposited in the joint cartilage and, less commonly, in the periarticular tissues. This entity and the differential diagnosis of chondrocalcinosis are described in Chapter 3.
Interstitial Calcinosis
Interstitial calcinosis is an uncommon condition in which there is either a localized or a widely disseminated deposition of calcium in the skin, subcutaneous tissues, muscles, and tendons. Calcinosis is often associated with collagen diseases, scleroderma, and dermatomyositis. However, it can exist in a relatively asymptomatic form with no signs of any associated disorder.
Calcinosis Universalis (Diffuse Calcinosis)
Calcinosis universalis is characterized by the wide dissemination of thin, calcific plaques of various sizes throughout the soft tissues, chiefly in the subcutaneous layer, and occasionally within the muscles and tendons. From a clinical point of view, there are several types of diffuse calcinosis. (1) An asymptomatic has been reported but is rare. (2) Diffuse calcinosis associated with generalized scleroderma (Fig. 10-20) includes the CREST syndrome (calcinosis, Raynaud’s phenomenon, esophageal hypomotility, sclerodactyly, and telangiectasia) and the Thibierge-Weissenbach syndrome (calcinosis and acrosclerosis). (3) Diffuse calcinosis is associated with dermatomyositis (Fig. 10-21).
In scleroderma and dermatomyositis, the calcification occurs in the form of thin plaques. In scleroderma, these are limited to the skin and immediate subcutaneous tissues; in dermatomyositis, calcification also occur in the muscles. The calcification appears in four distinct patterns: superficial masses, deep masses, deep linear deposits, and a lacy, reticular, subcutaneous deposition that encases the torso. This last pattern is of particular importance because it is associated with a steadily progressive deterioration and is therefore of prognostic significance. In addition to the plaques, there is a general loss of soft-tissue differentiation, with the subcutaneous fat layer becoming very scanty or disappearing altogether.
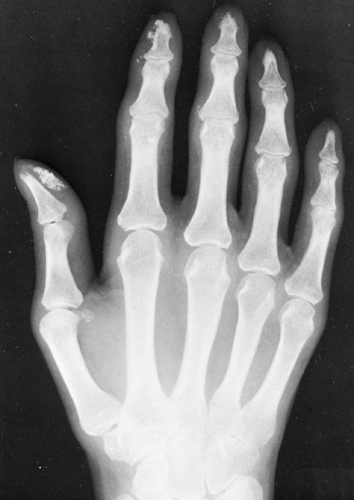
Calcinosis Circumscripta
In the localized type of calcinosis, the calcification occurs in the form of small, rounded foci having an amorphous appearance. These foci are found chiefly in the tips of the fingers and along the margins of the joints in the hands and feet. The changes are noted more frequently in the hands than in the feet and, when present in both areas, are usually more intensive in the hands. As is true with the diffuse form of calcinosis, the localized type occurs in several different clinical manifestations.
Without Associated Skin or Vasospastic Phenomena
These lesions appear most frequently in elderly persons and are more common in women than in men. There may be some aching in the joints of the affected areas. The foci can be numerous, and some may be sufficiently large to cause visible swellings. Ulceration of the skin occurs over the larger lumps in some of these patients and is followed by extrusion of cheesy, whitish material and subsequent healing.
With Associated Scleroderma
Calcinosis is common in patients with scleroderma affecting the fingers and hands. The calcification may appear as a few tiny, rounded, subcutaneous nodules or as larger masses. These are commonly found in the terminal phalanges or along the margins of the joints. Other roentgenographic signs of scleroderma often are present, including (1) diminution in the amount of soft tissues in the tips of the fingers, so that these digits develop a tapered or almost pointed appearance, and (2) absorption of bone. The latter begins in the terminal tufts of the distal phalanges of the affected fingers, so that the tufts disappear and the shaft of the phalanx becomes pointed. The absorption may extend to involve the shaft and can be sufficiently severe so that most of the bone disappears or fragments (Fig. 10-22).
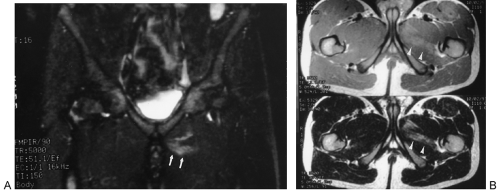
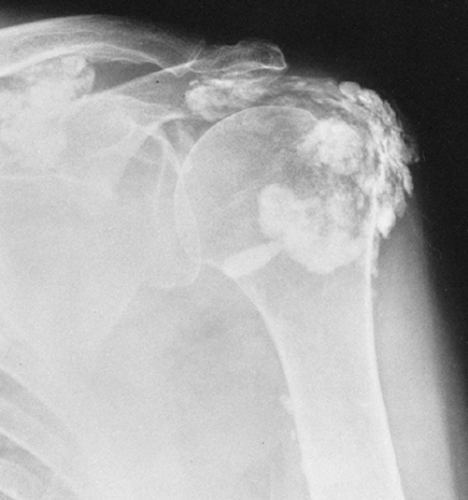
Tumoral Calcinosis
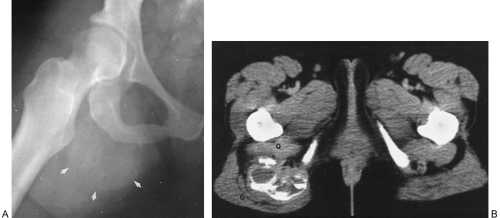
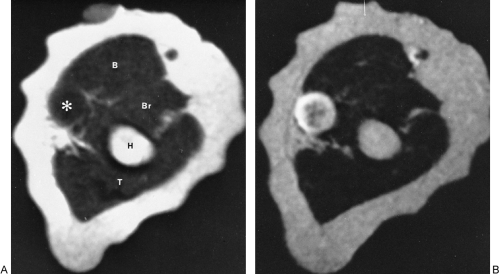
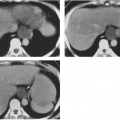
Tumoral calcinosis is a rare entity characterized by the occurrence of a large calcified mass, usually near one of the larger joints, particularly the hip.16,58 The hallmark is the presence of large, multiglobular, para-articular calcific masses (Fig. 10-23) usually related to the extensor surface of the involved joints. In order of decreasing frequency the
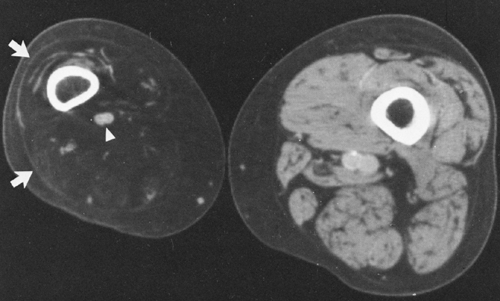
hips (Fig. 10-24), elbows, shoulders, and feet are affected. Multiple masses may be present. In general, the adjacent bone is not involved. Clinically there is pain and localized swelling. The condition is benign but often recurs after surgical removal. Roentgenographic findings are those of a round or oval, well-circumscribed mass of calcium, which may be lobulated. It appears in periarticular soft tissues and consists of multiple smaller opacities separated by radiolucent lines representing fibrous-tissue septa (Figs. 10-23 and 10-24A). Occasionally, fluid levels may be observed in upright films (Fig. 10-23). Fluid levels are also demonstrated by CT, which also shows that the calcific collections arise in the fascial planes between muscles. CT demonstrates this phenomenon quite nicely with dependent layering of the calcium and, less commonly, calcium lining a cyst-like structure (Fig. 10-24B).2
hips (Fig. 10-24), elbows, shoulders, and feet are affected. Multiple masses may be present. In general, the adjacent bone is not involved. Clinically there is pain and localized swelling. The condition is benign but often recurs after surgical removal. Roentgenographic findings are those of a round or oval, well-circumscribed mass of calcium, which may be lobulated. It appears in periarticular soft tissues and consists of multiple smaller opacities separated by radiolucent lines representing fibrous-tissue septa (Figs. 10-23 and 10-24A). Occasionally, fluid levels may be observed in upright films (Fig. 10-23). Fluid levels are also demonstrated by CT, which also shows that the calcific collections arise in the fascial planes between muscles. CT demonstrates this phenomenon quite nicely with dependent layering of the calcium and, less commonly, calcium lining a cyst-like structure (Fig. 10-24B).2
Deposits of calcium pyrophosphate within the medullary space of long bones in this condition have resulted in a painful, inflammatory response, a diaphysitis, manifested by surrounding periosteal reaction and changes in the marrow signal on MRI.58
Specific biochemical abnormalities have been identified in tumoral calcinosis, establishing it as a metabolic disease. These are hyperphosphatemia, elevated serum 1,25-dihydroxyvitamin D, and elevation of the renal phosphate reabsorption threshold. The serum calcium, parathyroid hormone, renal function, and alkaline phosphatase results are normal.
Similar masses of calcium have been found in association with pseudoxanthoma elasticum, a rare hereditary disorder characterized by degeneration of elastic tissue. The most common findings are xanthoma-like lesions of the skin. Arterial calcification is common. Multiglobular masses of calcium, indistinguishable from those of tumoral calcinosis, may be encountered in patients with chronic renal failure who are undergoing hemodialysis (Fig. 10-25).
MISCELLANEOUS FORMS OF SOFT-TISSUE CALCIFICATION
Subcutaneous and Intramuscular Calcification in Patients Receiving Calcium Gluconate Therapy
When calcium gluconate is administered intramuscularly to infants or when it extravasates during intravenous injection, a tissue reaction occurs. This results in hazy, amorphous calcification in muscle and subcutaneous tissues that is not produced by the administered calcium, since no roentgenographic findings are present initially. Erythema and induration develop, causing a hard mass that begins to calcify in a few days and then becomes visible radiographically (Fig. 10-26). The mass may continue to increase in size for about 2 weeks. There is then a gradual decrease in size, followed by eventual disappearance of the calcium. If large subcutaneous doses are administered, ulceration and extrusion of the calcium may occur. Calcification may also be observed in vessels in the area of extravasation.
Ehlers-Danlos Syndrome
The Ehlers-Danlos syndrome, a congenital dystrophy with hereditary and familial aspects, is a rare cause of disseminated
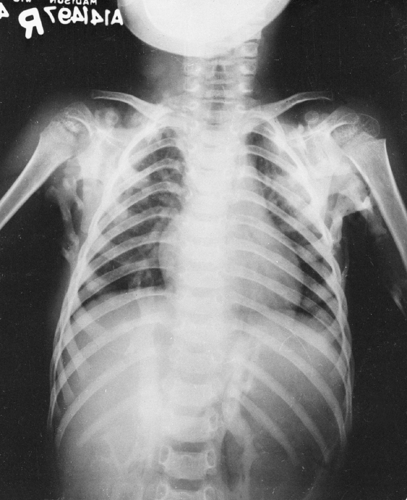
subcutaneous calcifications. It is characterized by an unusual hyperelasticity and fragility of the skin and blood vessels, hypermobility of the joints, pseudotumors over the bony prominences, and disseminated, movable subcutaneous nodules. The subcutaneous nodules may calcify. They appear as round, discrete densities, usually ring-like with a central zone of translucency, ranging from 2 to 10 mm in diameter. They occur most frequently over the bony prominences of the forearms and legs. Additional findings include a variety of thoracic deformities, among which are scoliosis, kyphosis, pectus excavatum, and subluxation of the sternoclavicular joints.
subcutaneous calcifications. It is characterized by an unusual hyperelasticity and fragility of the skin and blood vessels, hypermobility of the joints, pseudotumors over the bony prominences, and disseminated, movable subcutaneous nodules. The subcutaneous nodules may calcify. They appear as round, discrete densities, usually ring-like with a central zone of translucency, ranging from 2 to 10 mm in diameter. They occur most frequently over the bony prominences of the forearms and legs. Additional findings include a variety of thoracic deformities, among which are scoliosis, kyphosis, pectus excavatum, and subluxation of the sternoclavicular joints.
Calcification in Tumors
Calcification is not a specific finding for any single type of tumor of the soft tissues, with the exception of hemangioma. Calcification in the form of phleboliths is a frequent finding in cavernous hemangioma and is often diagnostic (see Fig. 10-14). In other tumors, calcification results usually because of a deficient blood supply, with subsequent necrosis within a solid, slowly growing neoplasm. Deposits of calcium may be seen most commonly in lipomas, liposarcomas, fibrosarcomas, and synovial sarcomas (see Fig. 3-50 in Chapter 3). Chondral calcification is seen in parosteal chondromas (see Chapter 4) and in the less common cartilage tumors of soft tissues. Rarely, osteosarcoma has been found arising in the soft tissues, as a result of cellular metaplasia.
GAS IN THE SOFT TISSUES
Accumulations of gas in the soft tissues can easily be recognized on roentgenograms because of the extreme radiolucency of gas compared with the opacity of the surrounding soft tissues. Occasionally, a localized deposition of fat (e.g., lipoma), may appear sufficiently lucent on roentgenograms with very high contrast to suggest the presence of a local pocket of gas. However, an accumulation of air or other gas of a size similar to that of a deposit of fat would appear
considerably darker, and it usually requires little experience to differentiate the two. Two major types of gas can be found in the soft tissues. One is air that may have gained entrance through a wound or surgical procedure. The other is gas that has been formed as a result of anaerobic bacterial action. Occasionally, in patients with massive pneumomediastinum, gas dissects into the peritoneum and into superficial soft tissues of the face, neck, thorax, and abdomen. The presence of any appreciable amount of subcutaneous air is termed subcutaneous emphysema.
considerably darker, and it usually requires little experience to differentiate the two. Two major types of gas can be found in the soft tissues. One is air that may have gained entrance through a wound or surgical procedure. The other is gas that has been formed as a result of anaerobic bacterial action. Occasionally, in patients with massive pneumomediastinum, gas dissects into the peritoneum and into superficial soft tissues of the face, neck, thorax, and abdomen. The presence of any appreciable amount of subcutaneous air is termed subcutaneous emphysema.
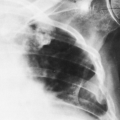
Subcutaneous Emphysema
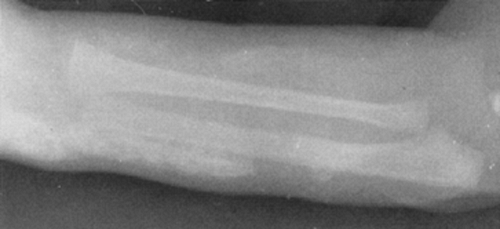
Small bubbles or streaks of air are frequently seen in the region of soft-tissue wounds of penetrating nature. The air shadows may persist for several hours or longer after the injury, but they usually disappear after 1 or 2 days. Subcutaneous emphysema may occur after injuries to the thorax, usually rib fractures (Fig. 10-27). There may or may not be an associated pneumothorax. Occasionally, after this type of injury, the subcutaneous emphysema becomes very extensive and the air extends widely through the fascial planes of the body. After surgical procedures on the thorax, fairly large amounts of subcutaneous emphysema may be seen for several days. Traumatic rupture of the trachea, larynx, or esophagus may also result in subcutaneous emphysema. Because of the free communication of the fascial spaces of the body, air may extend far from its point of origin. The recognition of subcutaneous emphysema is not difficult unless the amount of gas is small.
Gas Gangrene
Infection with gas-forming organisms may result in the roentgenographic visualization of bubbles or streaks of gas in the subcutaneous or deeper tissues. This may occur after penetrating or crushing injuries or surgical procedures. The organism most frequently found is Bacillus welchii (Clostridium perfringens). It is not possible to distinguish gas formed by anaerobic bacteria from air that has been introduced from without, and therefore the diagnosis of gas gangrene cannot be made from roentgenographic evidence during its very early stage. If the gas shadows extend for a considerable distance from the known site of the soft-tissue wound, infection can be suspected. If serial examinations over a period of hours or several days show definite evidence of increasing amounts of gas and spread of the gas shadows, the evidence for gas bacillus infection is more conclusive (Fig. 10-28).
In diabetic gangrene involving the foot, it is common to find small bubbles of gas in the region of the gangrenous tissue and occasionally even more extensively throughout
the soft tissues of the foot or in the distal part of the leg (see Fig. 5-16



the soft tissues of the foot or in the distal part of the leg (see Fig. 5-16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree