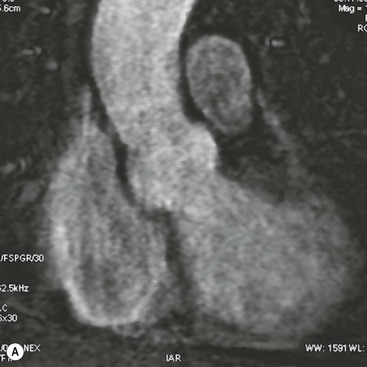
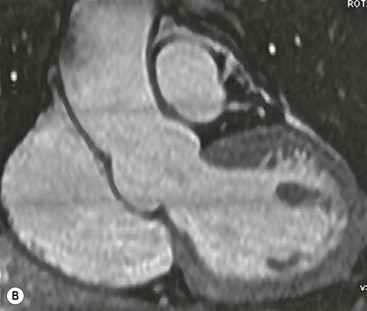
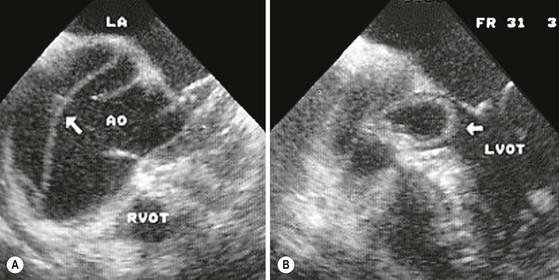
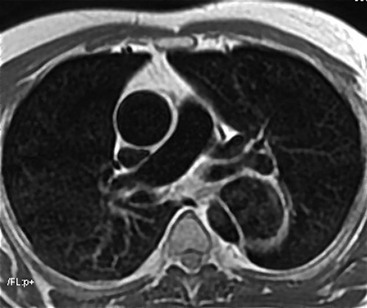
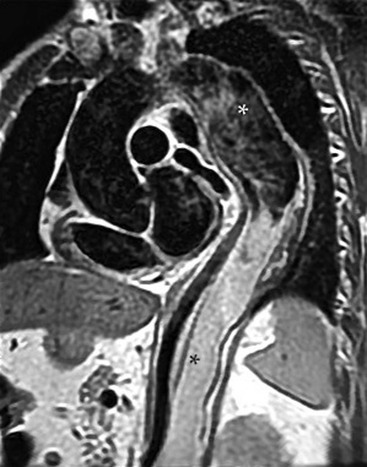
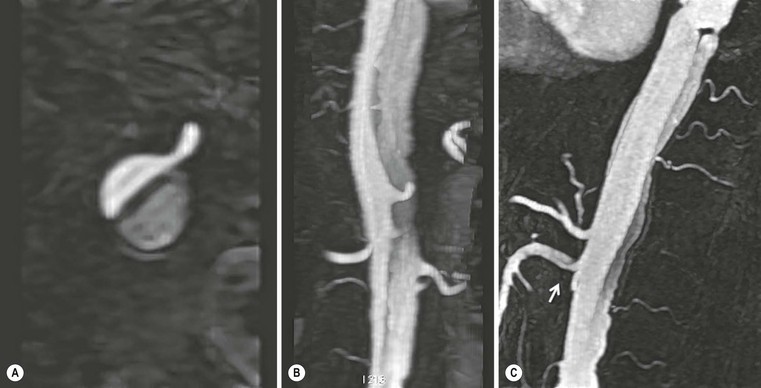
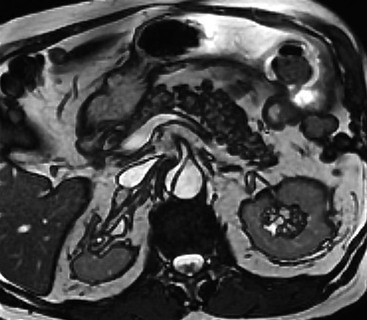
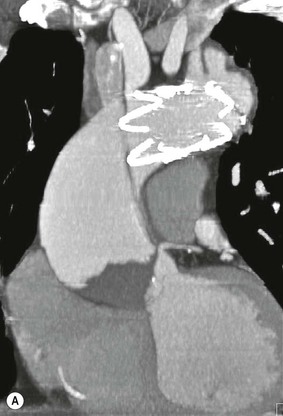
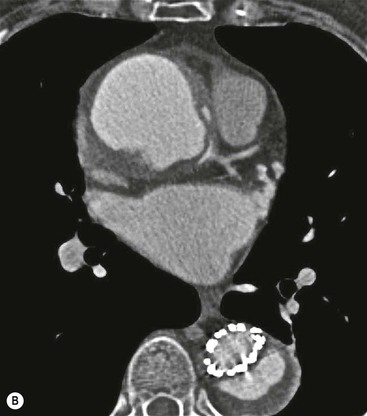
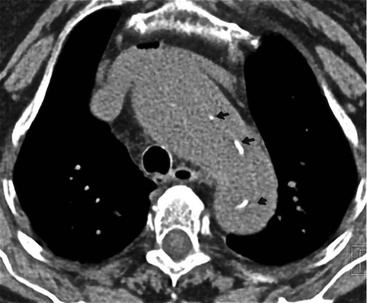
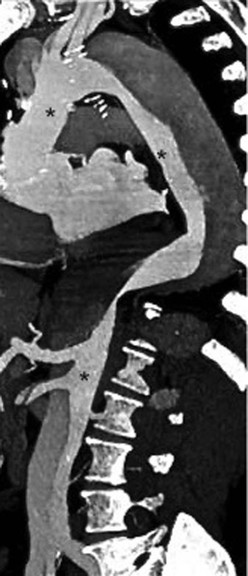
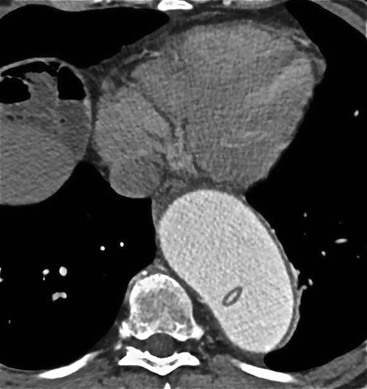
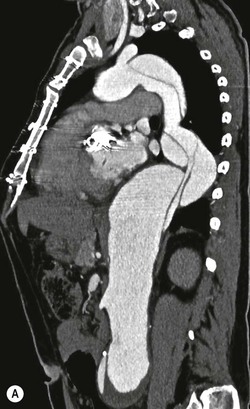
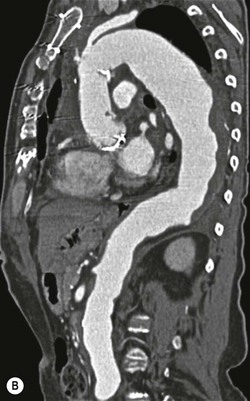
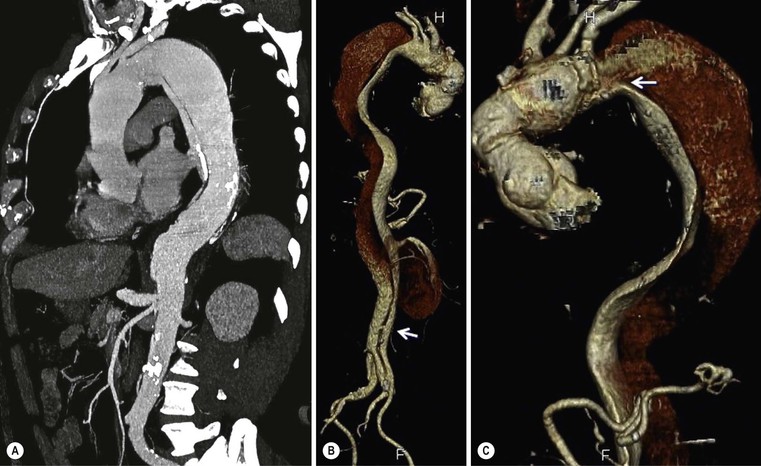
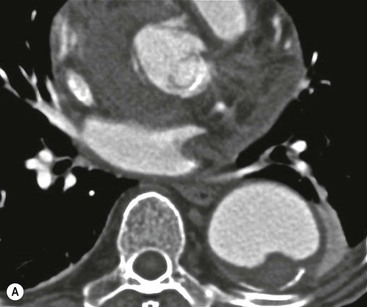
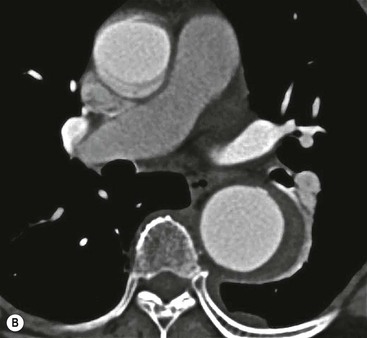
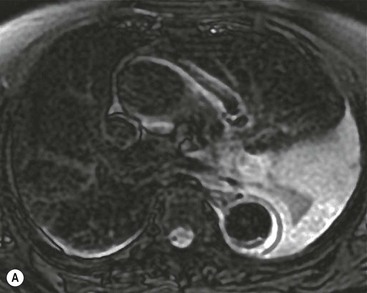
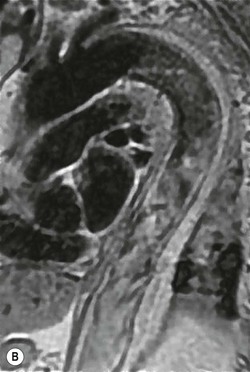
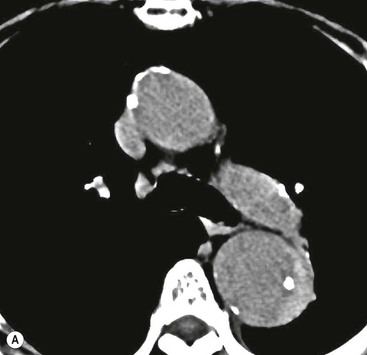
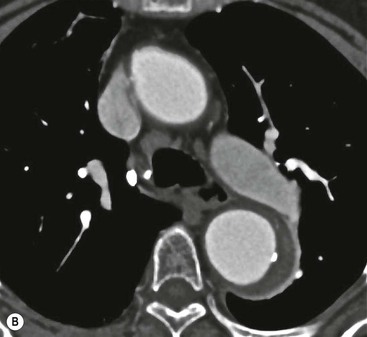
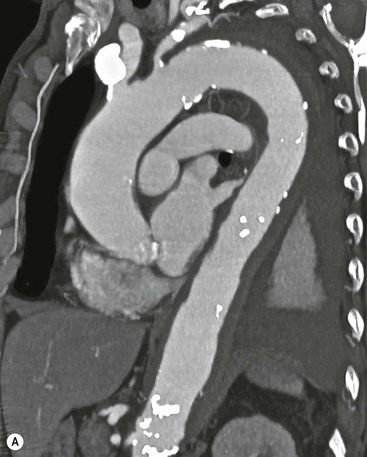
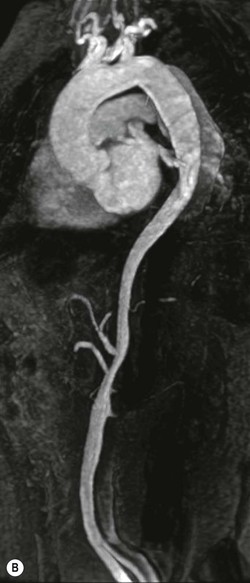
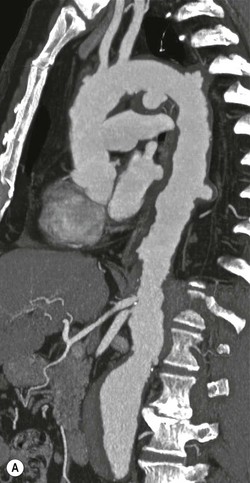
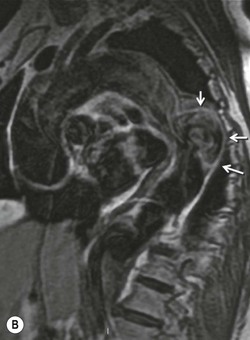
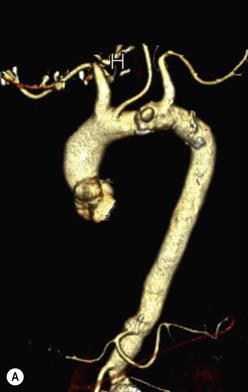
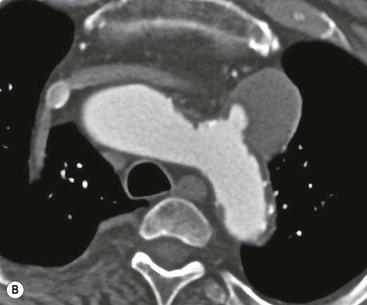
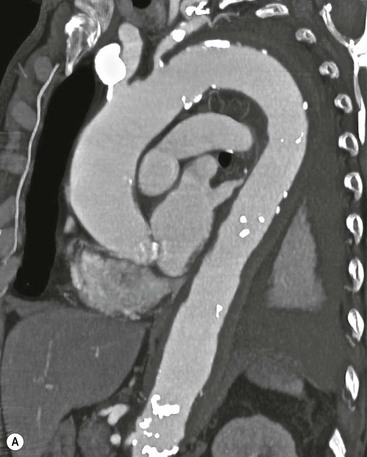
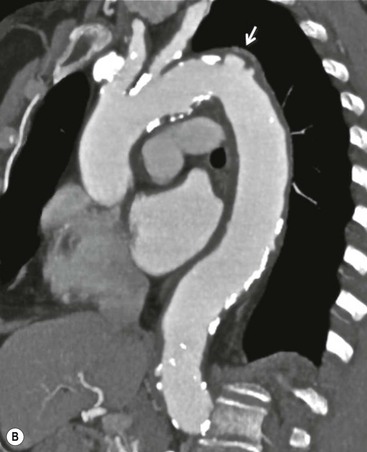
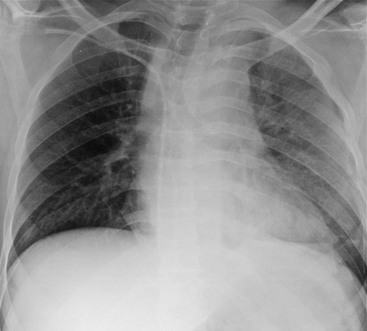
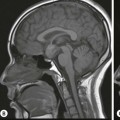
Rossella Fattori, Luigi Lovato The aorta is the main artery of the body delivering oxygenated blood from the left ventricle to all parts. In common with other arteries it has three histologically distinct layers: an intima consisting of a thin endothelial layer; a media containing an elastic lamella, smooth muscle and connective tissue; and a thin outer adventitia made of connective and elastic tissues also containing nerves, lymphatics and the vasa vasorum.1 The aortic root begins at the upper part of the left ventricle and is approximately 3 cm in diameter. A normal aorta passes superiorly and to the right for approximately 5 cm, then arches posteriorly over the root of the left lung, descending within the thorax beside the vertebral column, gradually achieving the median plane, and becoming the abdominal aorta, after it passes through the aortic hiatus in the diaphragm. The abdominal aorta is approximately 2 cm in diameter; it ends slightly to the left of the median plane at the lower border of the fourth lumbar vertebra by dividing into the right and left common iliac arteries. The aortic root and most of the ascending aorta is contained within the pericardium. The root consists of three sinuses: the right coronary artery arising from the right coronary sinus, the left coronary artery from the left coronary sinus and a non-coronary sinus which is usually located to the right and posterior. The ascending aorta forms the right mediastinal border on a postero-anterior (PA) chest radiograph. It becomes the aortic arch at the origin of the innominate artery and also gives rise to the left common carotid and left subclavian arteries. Around three-quarters of people show this ‘normal’ branch pattern of the supra-aortic arteries, but in 20% the innominate and left common carotid arteries have a common origin, and in 6% the left vertebral artery arises directly from the aortic arch. The aortic arch ends and the descending thoracic aorta begins immediately beyond the origin of the left subclavian artery. At this site the ligamentum venosum (the embryological ductus arteriosus which closes within a few days of birth) joins the inferior concavity of the aortic arch to the main pulmonary artery. The aorta is fixed at this point. Occasionally the duct may persist as a short diverticulum.2 The last few decades have seen an increasing recognition of thoracic aortic disease among Western people,3 partly due to greater longevity and an increased awareness of its clinical importance.4,5 Recent technological advances in CT and MRI have greatly contributed to the increased recognition and pathological understanding of aortic disease. There are at least three main goals of imaging concerning thoracic aortic diseases: disease recognition, preoperative evaluation and imaging follow-up. The appropriate imaging technique depends on which of these aspects is pre-minent (Table 24-1). Aortic disease often presents as a clinical emergency, with patients becoming rapidly haemodynamically unstable over time.6 Accordingly, non-invasiveness, diagnostic accuracy and speed are the main properties requested in this setting, together with a comprehensive evaluation of the thoracic aorta (crucial for an accurate assessment before any intervention). Thus the choice of the optimal imaging technique should consider these aspects and various patient-related factors (namely, acute or chronic presentation). Evaluation of the thoracic aorta has always been very difficult with first-line imaging techniques like chest X-ray (CXR) and transthoracic ultrasound (except for proximal aortic segments) due to its anatomical location. CXR may only identify indirect signs of aortic aneurysm or dissection such as a widening of the upper mediastinum or an abnormal aortic contour increase, but it lacks sufficient sensitivity to exclude significant aortic disease, especially in high-risk patients7 and, almost invariably, additional imaging is required for clarification. Moreover, CXR does not give any information about anatomical details for surgical or endovascular planning. Transthoracic echocardiography (TTE) is limited by its restricted field of view, further reduced by acoustic window limitations in adult patients (e.g. due to chronic pulmonary diseases, surgical scars or obesity), and has a typical ‘blind spot’ at the level of the proximal aortic arch due to superposition of air in the right bronchus. Transoesophageal echocardiography (TOE) is superior to TTE for thoracic aorta evaluation8 and it can be easily performed at the bedside. However, it is partially invasive and not well tolerated by patients. Though echocardiography is routinely used for follow-up of aortic root and proximal ascending aorta aneurysms in chronic diseases, its narrow field of view prevents a comprehensive evaluation of the thoracic aorta. Furthermore, operator dependence limits the overall accuracy. However, ultrasound still plays the main role for valvular assessment in thoracic aortic diseases (coexisting valvular disease or valvular involvement in type A aortic dissection or ascending aortic aneurysm), while intraoperative TOE is often fundamental for endovascular or surgical aortic treatment. In summary, CXR and ultrasound, although easy to perform, non-invasive and inexpensive, provide some helpful information, but alone they cannot provide comprehensive information about aortic disease. For many decades angiography was the only available imaging technique for diagnosis and preoperative evaluation of aortic diseases. It was intrinsically invasive, relatively costly, and needed a well-organised and experienced team. It only provided limited information about the aortic wall, because angiography provides only ‘luminographic’ data. Thus it provided limited information about an intramural haematoma (IMH) and could be misleading in aortic dissection with a complete false lumen thrombosis. Over the past 30 years, with the advent of computed tomography (CT) and magnetic resonance imaging (MRI) and their recent technological evolutions, angiography has become progressively abandoned by physicians for diagnostic purposes.9 Indeed, CT and MRI combine non-invasiveness with high spatial and temporal resolution and can provide infromation about the entire length of the thoracic or thoracoabdominal aorta. Multiplanar images allow precise measurements of aortic diameters, preferably taken perpendicular to the longitudinal axis in more than one plane to avoid source of errors. In fact, the aorta has such variable geometry that it cannot usually be entirely visualised in a single plane. MR angiography (MRA) and CT angiography (CTA) have further enhanced the non-invasive visualisation of vascular structures with a high degree of spatial and contrast resolution in all three dimensions. Different 2D and 3D processing techniques, such as multiplanar reformation (MPR), maximum intensity projection (MIP) and volume rendering (VR), play an important role for preoperative planning.10 While thin-slice MPRs in any arbitrary plane provide high anatomical resolution, they cannot visualise the entire aorta in a single plane. MIP images of appropriate slab thickness, while demonstrating the whole aorta, only yield information of perfused lumina similar to digital subtraction angiography; as a threshold technique, MIP images may not discriminate lower-density intraluminal structures such as thrombus, plaque or intramural haematoma. MIP images also do not provide any fore- or background information, thereby limiting the perception of interstructural relationships. VR is a different 3D reconstruction technique where all tissues can be simultaneously represented. Using adequate filters, metallic stents or clips do not create artefacts and aortic wall lesions can be differentiated from the lumen. VR provides 3D anatomical information displaying the spatial relationship between aortic lesions and branching vessels. Finally, curved reformations can reconstruct even the most tortuous vascular structure in a single plane. All these processing and display options make CT and MRI measurements highly reproducible and less operator-dependent than ultrasound. CT and MRI currently form the backbone of thoracic aortic imaging. Both techniques show comparable results in terms of diagnostic accuracy, measurement reproducibility and anatomical detail definition. MRI does not use ionising radiation and gadolinium is less nephrotoxic than iodinated contrast medium. Consideration should only be given to patient with severe renal dysfunction (creatinine clearance <30 mg/dL/min) where it is necessary to balance the clinical usefulness of gadolinium and the risk of nephrogenic renal sclerosis.11 Recently, the development of 3D steady-state free-precession (SSFP) sequences with navigator echo seems to overcome this limitation,12 allowing MRI to obtain angiographic images of the aorta without gadolinium administration with similar accuracy compared to traditional MRA imaging (Fig. 24-1). The main limitation of CT imaging is the potential radiation risk,13 especially in neonates, children and young adults. Although recent CT technologies (dual-source CT, prospective gating, etc.) are reducing radiation exposure,14 the dose cannot be considered negligible, especially for repeated follow-up examinations of chronic aortic disease. Moreover, the use of iodinated contrast medium requires careful consideration in patients with borderline renal insufficiency or a history of previous reactions. Disease presentation is another aspect that influences the choice of imaging investigation. Thoracic aortic diseases frequently present acutely. MRI requires longer examination times, is less readily available and usually with less favourable logistics/locations; these factors often make it less suitable for patients with acute disease. CT is rapid and very accurate, and is particularly well-suited for the diagnosis and correct treatment planning of acute aortic problems. Modern MDCT allow for sub-millimetre, isotropic 3D data acquisition of extended anatomical ranges within comfortable (4–8 s) breath-hold duration; dedicated software provides 2D and 3D artefact-free reconstructions from virtually any angle and in any desirable plane. The introduction of the ECG gating15 has minimised motion artefacts, increasing the sensitivity and specificity for type A aortic dissection, especially with respect to identifying intimal flaps and aortic valve morphology. CT equipment is more widely available and often located close to the intensive care units or operating rooms. Moreover, when acute aortic dissection is suspected, MDCT is also able to exclude other potential causes of thoracic pain such as pulmonary embolism, other pulmonary diseases or coronary arteries disease16 with high accuracy, though this requires choosing appropriate examination protocols. Thus MRI, though highly accurate for acute aortic disease evaluation, is usually confined to cases of severe renal insufficiency or absolute contraindications to iodinated contrast medium. The high diagnostic accuracy of MR in patients with acute aortic syndrome is based on its high contrast resolution between the lumen and the vessel wall provided by black-blood fast spin-echo (BBFSE) and SSFP imaging, which allows differentiation of aortic wall alterations (e.g. intramural haematoma) from atheromatous plaques or aortitis. In patients with chronic aortic disease, follow-up and identification of findings requiring surgery are the main goals for imaging. Both CT and MRI provide highly reproducible and accurate aortic measurements, but MRI may be preferred to CT to minimise radiation exposure, especially in young adults affected by congenital aortic anomalies and genetic syndromes associated with thoracic aneurysms like Marfan’s or Turner’s syndrome. MRI, with cine gradient-echo sequences and phase contrast imaging, can also obtain functional informations on the thoracic aorta and quantify aortic valve regurgitation or stenosis within the same examination. CT is preferred to MRI only for aortic stent imaging follow-up. The stent material causes artefacts on MRI images, which reduce MRI’s sensitivity for detection of small endoleaks or stent structure alterations. The term ‘acute aortic syndrome’ comprises all aortic diseases characterised by a sudden clinical presentation that require acute hospitalisation (within 15 days from symptom onset) and often need urgent surgical or endovascular repair. It includes aortic dissection, IMH, penetrating aortic ulcer, traumatic aortic rupture, suture dehiscence and ruptured aneurysm. Aortic dissection is the most common non-traumatic acute aortic emergency with an overall in-hospital mortality of 20–25%, which increases markedly in patients with complicated dissection. The aetiology is frequently unknown, but is related to advancing age and hypertension. Cystic medial degeneration in connective tissue disorders such as Marfan’s syndrome and Ehlers–Danlos syndrome is a predisposing factor, as are coarctation, bicuspid aortic valve, aortitis, pregnancy and blunt chest trauma. Aortic dissection is initiated by an intimal tear, which allows blood to penetrate into the medial layer, producing a cleavage plane (false lumen) between the inner two-thirds and outer one-third of media. The true lumen is separated from the false lumen by an intimomedial flap. The blood course through the medial layer can variably extend the dissection distal or proximal to the entry tear and eventually rupture through the adventitia or back through the intima into the true lumen, creating re-entry tears. Branching vessels can be involved by the dissection process and may variably originate from the true lumen, the false lumen or both. The false lumen may thrombose completely or partially over time, while reduction in the amount of elastic tissue within the wall of the false lumen may lead to subsequent aneurysmatic dilatation. In the literature, there are various classification systems for aortic dissection, depending on the extent of the thoracic aorta involved (Table 24-2). The most frequenly used is the Stanford classification whereby an aortic dissection is called type A if it involves the ascending aorta, regardless of the site of entry tear. Type B dissections only involve the aorta beyond the left subclavian artery. This classification is focused on prognosis and strongly influences treatment approach, because, if not surgically treated, type A dissection leads to death in the majority of cases, while type B dissection can be successfully managed with medical therapy. Even type B dissections, when unstable, may require intervention to avoid severe complications and an increasing mortality. The anatomy of the dissection indicates the type of surgery or endovascular technique and their feasibility, thus affecting the procedure success rate and the long-term results. Therefore, the goal of imaging is not only the identification of the intimal flap and the extent of dissection but also a clear definition of the presence and site of entry and re-entry tears, the relationship between true and false lumen and the aortic branches (visceral, epiaortic, iliac and femoral vessels), the presence and degree of aortic valve or coronary artery compromise. Aortography was traditionally the gold standard in suspected aortic dissection even though catheter manipulation and directly high-flow contrast injection in a dissected aorta increased the risk of acute complications. The advent of non-invasive imaging also demonstrated its suboptimal accuracy, especially in terms of sensitivity (77 to 90%), while specificity was 90 to 100%. CT, MRI and TOE have rapidly substituted invasive angiography.17 TOE can be performed at the bedside in patients too unstable for transportation and can give haemodynamic information about flow in the true and false lumen, with excellent sensitivity for the aortic dissection confirmation (85–90%). TOE also provides valuable information about the functional status of the aortic valve and can assess the degree of involvement of the coronary arteries (Fig. 24-2) in type A dissection. However, being operator-dependent, its specificity is reduced in the ‘blind areas’ of the distal ascending aorta and the aortic arch. Besides, TOE cannot assess abdominal aorta and its visceral branches so, in stable patients a second imaging test is advisable for a comprehensive evaluation of the thoracoabdominal aorta. Intravascular ultrasound (IVUS) at 12.5 MHz provides intraluminal cross-sectional images of vessels and is able to demonstrate the entry tear and extent of dissection, but is particularly useful in differentiating the true and false lumen, and demonstration of dynamic obstruction. However, the transducers are single use only and expensive, most departments have limited experience of IVUS as a diagnostic tool and its role is almost exclusively confined to interventional applications.18 MRI is one of the most accurate tools for aortic dissection evaluation, with excellent sensitivity and specificity that approximate 100%.19 On MRI images the excellent contrast between the lumen and the aortic wall make the detection of the intimal flap very easy. In BBFSE sequences it appears linear inside the black vessel lumen (Fig. 24-3). The false lumen can be differentiated from the true lumen by its higher signal intensity due to the slower flow (Fig. 24-4). The presence of cobwebs adjacent to the outer wall, which represent dissected media residual strands, are also useful for identifying the false lumen. A fast spin-echo sequence in the sagittal plane should be performed to define the extension of the dissection in the thoracic and abdominal aorta, including the aortic arch branches (Fig. 24-4). The accurate definition of the anatomical details of the dissection (extension, site of entry and re-entry tear, aortic branch relationships) relies on gadolinium-enhanced 3D MRA and its reformatted images, also including a complete analysis of axial MRA images to confirm and improve spin-echo information (Fig. 24-5). The SSFP technique can generate images with a high-contrast resolution between the aortic lumen and the wall that may be used for morphological (2D or 3D images) or functional (cine sequences) analysis (Fig. 24-6). Cine sequences may be used as an additional tool to evaluate aortic valve involvement and to define the presence of an entry tear (flow turbulence). With more recent equipment 2D images can achieve a complete study of the thoracic aorta within a few minutes20 while a patient’s ECG, blood pressure and oxygen saturation can be monitored even during assisted ventilation. A 3D sequence may be an alternative to MRA in rare cases when gadolinium is contraindicated. Despite these strong capacities, which virtually make MRI an ideal imaging modality for aortic dissection, its use in acute dissection is very low,17 because of its unfavourable logistics and restricted availability that limits its use in emergency conditions. However, CTA, using modern MDCT equipment, achieves high image quality with much shorter acquisition times. In a few seconds MDCT can acquire a volume of coverage from supraortic vessels to the femoral arteries as an aortic dissection study requires, while improved temporal resolution and ECG gating acquisition minimise pulsation artefacts at the aortic root, with consequent sensitivity and specificity now approaching 100%21 (Fig. 24-7). The wide availability, the fast acquisition and reporting times and the high accuracy make MDCT the imaging method of choice for the diagnosis of aortic dissection. Unenhanced CT may demonstrate internal displacement of intimal calcifications (Fig. 24-8). Contrast-enhanced CT allows detection of the intimal flap as a thin linear low attenuation separating the contrast-enhanced true and false lumen. Injection of contrast medium via the left upper limb should be avoided as the very high attenuation from contrast medium within the left brachiocephalic vein can produce streak artefact across the aortic arch, potentially causing diagnostic difficulties. The differentiation of the false lumen from the true lumen is essential; a number of imaging findings can be helpful, like the cobweb sign, described before. In most cases the true lumen is smaller and more medially located one. On most contrast-enhanced CT images it may be identified by its continuity with the undissected portion of the aorta (Fig. 24-9). An unusual type of aortic dissection is the intimo-intimal intussusception produced by circumferential dissection of the intimal layer, which subsequently invaginates like a wind sock; CT shows one lumen wrapped around the other one, with the inner lumen invariably being the true one (Fig. 24-10). A dissection with thrombosed false lumen should not be confused with an aneurysm with calcified mural thrombus. High attenuation within the false lumen on unenhanced images can help to identify the former. In addition, a dissection tends to spiral as it passes along the aorta, whereas a thrombus maintains a constant relationship with the aortic wall. A mural thrombus arising in an aneurysm also tends to have a more irregular internal border whilst a dissection has a smooth internal border. Finally, calcification of the intima may be identified at the periphery of the thrombus in an aneurysm (Fig. 24-11). Visceral and supra-aortic vessel involvement can account for high mortality and MDCT can reliably diagnose these aspects, also with the use of MPR reconstructions. MIP and VR images greatly contribute to surgical planning and aortic segments measurements. VR is preferred to MIP as it preserves the variable enhancement patterns of the lumina and is more sensitive for visualisation of the flap (Fig. 24-12). IMH was first described in 1920 as ‘dissection without intimal tear’,22 but clinical recognition of this pathological entity except for autopsy or surgical specimen was almost completely absent before CT and MRI. In fact IMH is a lesion confined to the aortic wall and invasive angiography is not able to detect it. It is considered the consequence of a hypertensive rupture of the vasa vasorum within the medial layer and eventually results in a circumferentially oriented blood collection. IMH may occur spontaneously, but may also arise from a penetrating aortic ulcer in a severely atheromatic aortic wall. It has also been described following trauma. An IMH is visualised by cross-sectional imaging as an aortic wall thickening, symmetric or asymmetric, variable in thickness from 3 mm to more than 1 cm, and it must be differentiated from mural thrombus or plaque. Intimal displacement of calcification can aid in distinguishing these entities, because the IMH is a subintimal lesion, with calcifications displaced on top of the lesion facing the lumen. Acute IMH is hyperdense on unenhanced CT. Another difference is the shape of the aortic wall thickening: in IMH the borders are generally smooth, while a thrombus or a plaque are typically characterised by irregular margins (Fig. 24-13). With TOE, false-positive and false-negative diagnoses have been reported. In comparison to other imaging techniques, MRI had the best sensitivity for detecting IMH before the advent of MDCT.23 T1-weighted images reveal a typical crescent-shaped area of abnormal signal intensity within the aortic wall. MRI is also the only imaging modality able to assess the age of haematoma, exploiting the influence on MRI signal intensity of the different degradation product of haemoglobin: in the acute phase (0–7 days after the onset of symptoms) on T1-weighted spin-echo images, oxyhaemoglobin shows intermediate signal intensity whereas in the subacute phase (> 8 days), methaemoglobin shows high signal intensity. However, when the signal intensity is medium to low, it can be difficult to distinguish IMH from mural thrombus. T2-weighted spin-echo sequences may help in differentiating the two entities: signal intensity is high in recent haemorrhage but low in chronic thrombosis (Fig. 24-14). MDCT, like MRI, has proven to be highly accurate in the diagnosis of IMH, with comparable sensitivity and specificity. In the suspect of IMH it is important to perform unenhanced CT: in the acute phase the haematoma appears as a crescent-like aortic wall thickening typically hyperdense on unenhanced CT with respect to the aortic lumen, while after enhancement the density of wall and lumen are reversed, with the IMH remaining unenhanced, unlike the false lumen in aortic dissection (Fig. 24-15). The differentiation between IMH and a completely thrombosed false lumen may be very difficult and the following findings are useful for differential diagnosis: IMH maintains a constant circumferential relationship to the wall (subintimal lesion), while the thrombosed false lumen tends to longitudinally spiral around the aorta. Secondly, IMH does not reduce the lumen, which maintains its regular shape, while the false lumen can variably compress the true lumen (Fig. 24-16). MDCT is highly accurate for the detection of small circumscribed intimal defects that can appear at multiple levels of the IMH; they can enlarge over time, evolving towards aneurysmatic dilation, and may eventually represent a patient subgroup with worse prognosis.24 The diagnosis of IMH is mainly based on axial images, but 2D reformatted images may be useful to evaluate the extent of IMH and its relationships with aortic branches. MRA, like conventional angiography, has poor value in IMH diagnosis because it provides only luminal information. An appropriate adaptation of the window level in reformatted images can help identify the wall haematoma. An aortic ulcer is generated by erosion of an atheromatous plaque disrupting the internal elastic lamina, exposing the media to pulsatile arterial flow and subsequent haematoma formation. This is distinguished from an atheromatous plaque by the presence of a focal, contrast medium-filled outpouching surrounded by an IMH. An atheromatous plaque does not extend beyond the intima, is frequently calcified and lacks an IMH. The extension of the ulceration to the medial layer can also evolve in localised dissection or even break through into the adventitia, creating an aortic pseudoanurysm. If the adventitia ruptures, only the mediastinal tissue can contain the haematoma; otherwise, the rupture is complete. PAUs are mainly located in the descending aorta but may be also seen in the aortic arch. Ulcers can be multiple and are frequently associated with a severe atherosclerotic aortic wall. The imaging diagnosis of PAU is based on the visualisation of a crater-like, contrast-filled outpouching with jagged edges, of variable extension, which may result in a large pseudoaneurysm25 (Fig. 24-17). Mural thickening can be associated (localised haematoma) as well as aortic dissection. Differently from IMH, conventional angiography has a good sensitivity for PAU, but both CT and MRI are better suited to evaluate the presence of associated lesions like atherosclerotic disease extent, localised haematoma and dissection. Unenhanced CT has the advantage of visualising intimal calcification displacement. MRA and CTA, including 2D and 3D reformatted images, are important for analysing the often-complex spatial relationships between the ulcers and the aortic branches (Fig. 24-18). The various aortic diseases as described so far are related to each other: PAU and IMH are both potential precursors of dissection.25 As they are lesions that involve a more external portion of the aortic wall, they are more prone to rupture than aortic dissection and need careful monitoring in the acute phase.26 Moreover, IMH can evolve into PAU (Fig. 24-19). Traumatic aortic injury can result from both penetrating and blunt chest injuries. Motor vehicles accidents are one of the main causes of TAI. In the United States there are around 40,000 motor vehicle deaths yearly and it has been estimated that 20% of deaths are caused by aortic rupture. A number of mechanisms with an increased risk for TAI have been proposed, of which the most important is rapid and sudden deceleration during the impact. The aortic segment subjected to the greatest strain is the isthmus, where the relatively mobile thoracic aorta is fixed by the ligamentum arteriosus: 90% of aortic ruptures occur here. Another mechanism leading to TAI consists of torsion caused by displacement of the heart to the left during antero-posterior compression, which typically involves the ascending aorta close to the innominate artery or immediately superior to the aortic valve; this is seen with vertical deceleration caused by falls from large heights. Other aortic segments are less commonly involved like the distal descending (diaphragmatic) aorta or the abdominal infrarenal segment, suggesting underlying mechanisms other than the sudden deceleration strain: one process refers to the ‘osseous pinch’, leading to compression of the heart and aorta between the sternum and vertebral column with a trauma extended from the intima to the adventitia (Table 24-3). In the majority of patients (80–90%) there is complete rupture of the aorta, with death occurring at the scene of the accident. Those patients that reach the hospital alive have injuries that vary from a simple intimal lesion, an IMH to a false aneurysm when the laceration extends through the media into the adventitia (which may be the only layer maintaining aortic integrity). Periaortic haemorrhage is frequently seen irrespective of the type of lesion. TABLE 24-3 Presley Trauma Center CT Grading System: Grades and CT Findings Modified from Gavant (1999).56 The clinical diagnosis of TAI can be difficult due to the lack of specific symptoms or signs in many patients. CXR gives only indirect signs of an aortic lesion like haemomediastinum (Table 24-4), which is insufficiently specific and is more likely the consequence of venous bleeding relative to the thoracic trauma. Moreover, chest radiographs in patients with suspected TAI are taken in the supine position, so the interpretation of mediastinal widening can be problematic—especially in obese patients. Upper limits for a normal mediastinal width or the ratio of the mediastinal width to chest width at the level of the aortic arch (M/C ratio) have been proposed (8 cm and 0, 25, respectively) but have a wide range of reported sensitivities and specificities. In any case, the initial CXRs performed as part of a trauma series may suggest an aortic involvement with satisfactory sensitivity (80–90%), showing the displacement of the nasogastric tube by the haematoma (Fig. 24-20). This makes chest radiography a useful screening tool for mediastinal haemorrhage, even though a normal mediastinum does not exclude a significant aortic injury, as seen in 7.3% of patients with subsequent proven TAI. TABLE 24-4 Chest Radiograph Findings Associated with Traumatic Aortic Injury * Most commonly seen signs. In most of the cases, the mechanism of trauma is the crucial element that alerts the clinician to the possibility of TAI: a high degree of suspicion should be raised in case of road traffic accidents (RTAs) at speeds greater than 30 mph, falls from greater than 10 ft and severe crush injuries to the chest. Injuries to other organs are commonly associated, detracting attention from a possible TAI, strongly affecting prognosis and treatment, but overall deeply influencing the choice of the imaging approach, which often depends on the haemodynamic stability and the need of concomitant injuries diagnosis.
The Thoracic Aorta
Diagnostic Aspects
The Normal Aorta
Diagnostic Aspects
Acquired Aortic Abnormalities
Acute Aortic Syndrome
Aortic Dissection
Classification
Imaging
Intramural Haematoma
Penetrating Atherosclerotic Ulcer (PAU)
Traumatic Aortic Injury (TAI)
Grade
Subgrade
CT Findings
Grade I
Normal aorta
Ia
Normal thoracic aorta
No mediastinal haematoma
Ib
Normal thoracic aorta
Mediastinal haematoma (para-aortic)
Grade II
Minimal aortic injury
IIa
Small (<1 cm) pseudoaneurysm
Indeterminate <1 cm intimal flap or thrombus
No mediastinal haematoma
IIb
Small (usually <1 cm) pseudoaneurysm
Indeterminate (<1 cm) intimal flap or thrombus
Mediastinal haematoma (para-aortic)
Grade III
Confined thoracic aortic injury
IIIa
>1-cm regular, well-defined pseudoaneurysm
Intimal flap or thrombus
No ascending aorta, arch or great vessel involvement
Mediastinal haematoma
IIIb
>1-cm regular, well-defined pseudoaneurysm
Intimal flap or thrombus
Ascending aorta, arch or great vessel involvement
Mediastinal haematoma
Grade IV
Total aortic disruption
IV
Irregular, poorly defined pseudoaneurysm
Intimal flap or thrombus
Mediastinal haematoma

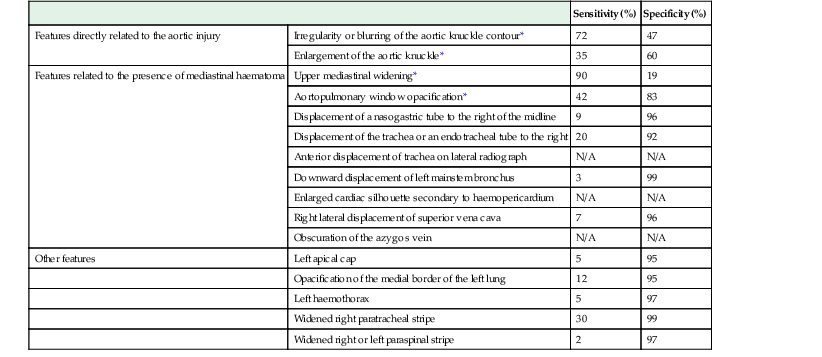
Imaging
Sensitivity (%)
Specificity (%)
Features directly related to the aortic injury
Irregularity or blurring of the aortic knuckle contour*
72
47
Enlargement of the aortic knuckle*
35
60
Features related to the presence of mediastinal haematoma
Upper mediastinal widening*
90
19
Aortopulmonary window opacification*
42
83
Displacement of a nasogastric tube to the right of the midline
9
96
Displacement of the trachea or an endotracheal tube to the right
20
92
Anterior displacement of trachea on lateral radiograph
N/A
N/A
Downward displacement of left mainstem bronchus
3
99
Enlarged cardiac silhouette secondary to haemopericardium
N/A
N/A
Right lateral displacement of superior vena cava
7
96
Obscuration of the azygos vein
N/A
N/A
Other features
Left apical cap
5
95
Opacification of the medial border of the left lung
12
95
Left haemothorax
5
97
Widened right paratracheal stripe
30
99
Widened right or left paraspinal stripe
2
97
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Thoracic Aorta
Chapter 24