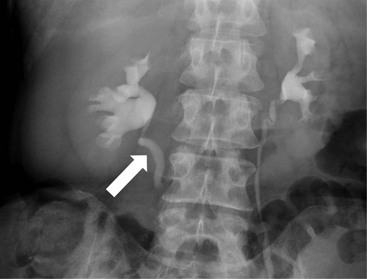
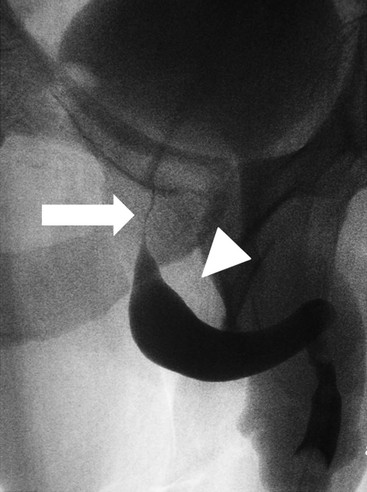
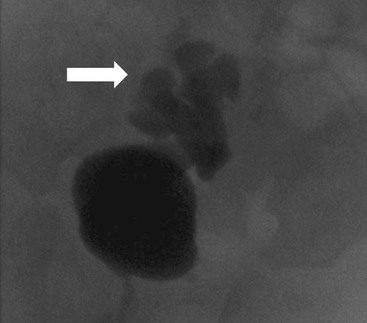
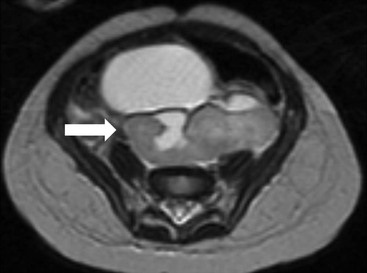
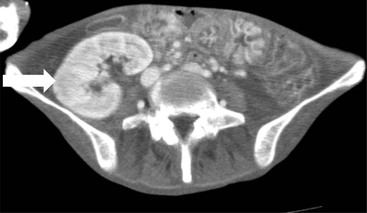
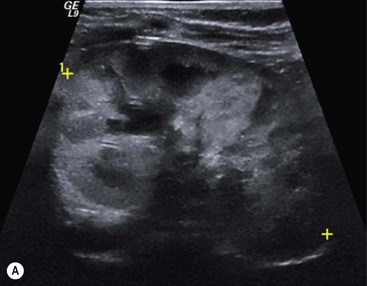
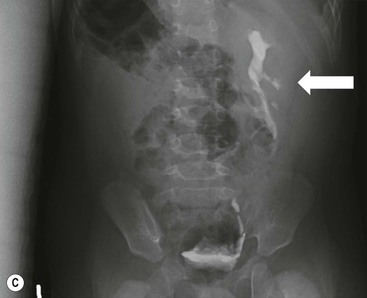
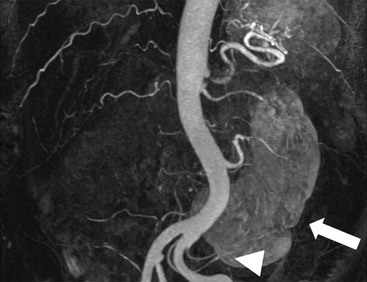
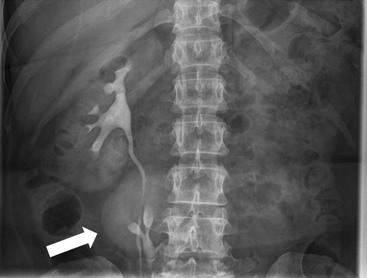
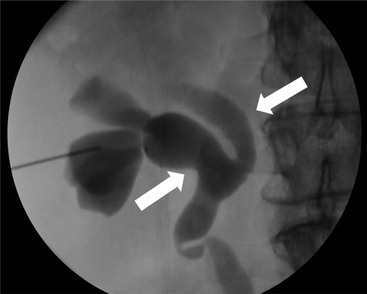
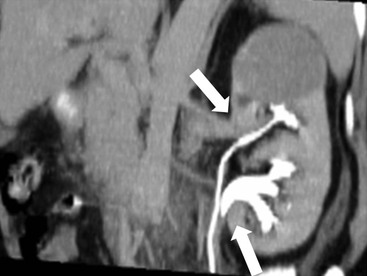
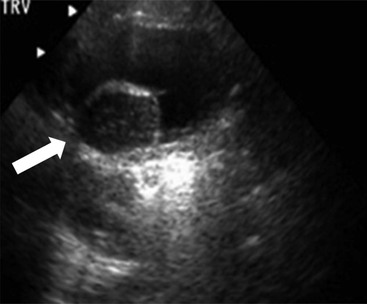
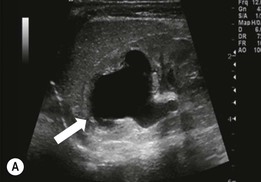
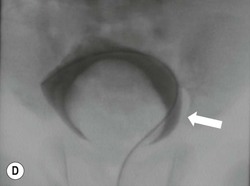
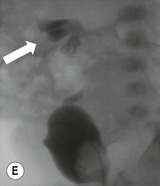
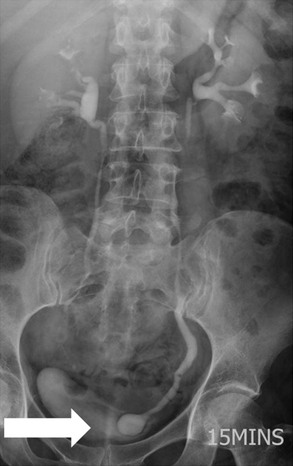
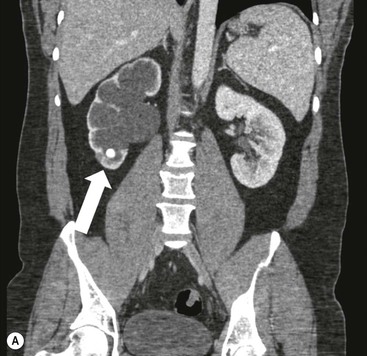
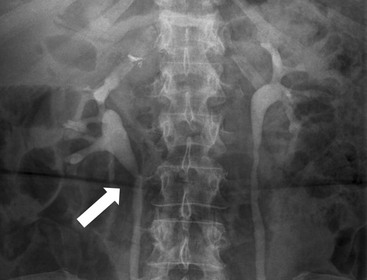
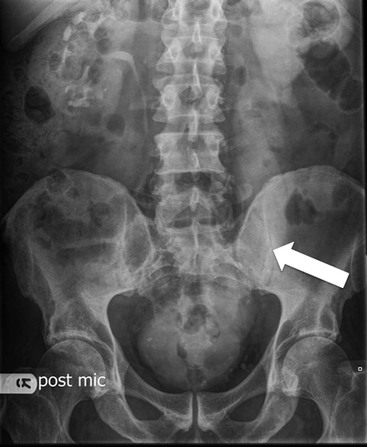
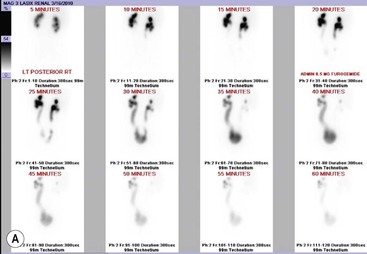
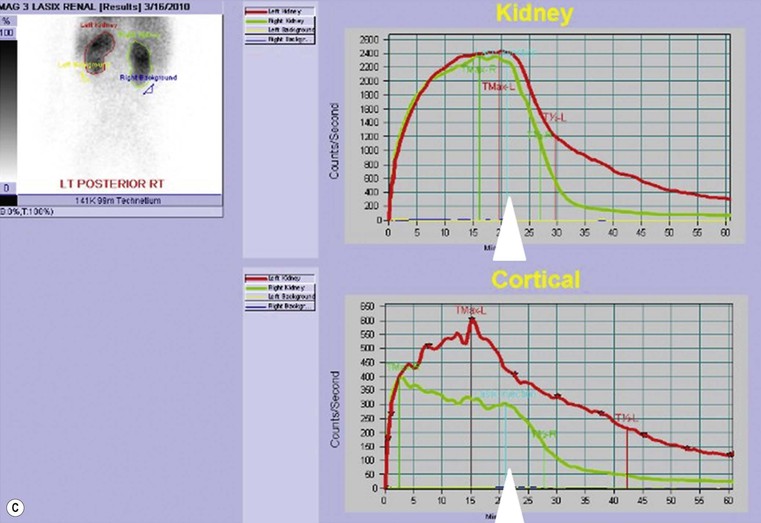
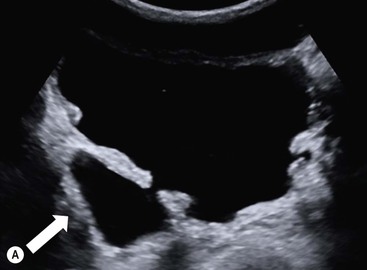
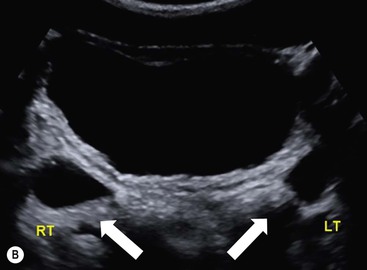
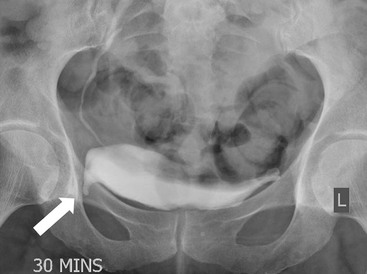
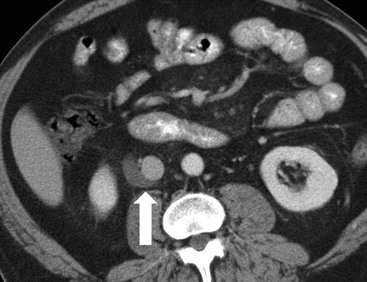
Owen J. O’Connor, Michael M. Maher This chapter describes clinically important aspects of urinary tract imaging with specific reference to anatomy, techniques and radiation. We begin with an outline of urinary tract embryology in order to place urinary tract anatomy and many anatomic variations into context. We describe long-established techniques of urinary tract imaging and the principles used to guide development of newer techniques. Imaging of the urinary tract is an evolving process, which has seen the introduction of CT urography in recent years and also in recent efforts aimed at radiation dose reduction. Current low-dose imaging practices are described. An understanding of urinary tract development helps foster better appreciation of urinary tract anatomy and the anatomical variation and anomalies that are encountered. Congenital urinary tract anomalies have a prevalence of approximately 10%. Due to complex inter-relationships during development, once one anomaly occurs, there is a 75% chance of another congenital anomaly either in the urinary tract, genital, musculoskeletal, gastrointestinal or cardiovascular system. The kidneys develop from primitive excretory organs, which emerge in a craniocaudal sequence beginning in the cervical region of the fetus at three weeks’ gestation. Renal development begins with a transient precursor organ called the pronephros, which is later replaced by the mesonephros at four to eight weeks’ gestation. Although most of the mesonephros degenerates, a portion develops into the mesonephric or Wolffian duct, which gives rise to the ureteric bud and male genital development. The ureteric bud emerges from mesonephric duct close to its insertion with the cloaca.1 The segment of the mesonephric duct between the cloaca and ureteric bud origin is known as the common excretory duct.2 The proximal ureteric bud enlarges into an ampulla, which undergoes dichotomous division to form the renal pelvis, calyces and collecting tubules of the renal medulla.3 This results in the development of 10 to 14 minor calyces. Since the interpolar calyces are initially formed, the polar calyces are more likely to have fewer calyces or have incomplete divisions. The kidney itself develops from the metanephros, which is initially located in the sacral region of the fetus. The metanephric blastema forms the renal excretory ducts (proximal and distal convoluted tubules, loop of Henle), interstitium of the renal parenchyma, as well as Bowman’s capsule, once contact is made with the ureteric bud.4 The bladder develops following craniocaudal division of the cloaca into the urogenital sinus anteriorly and the anorectal portion posteriorly by the urorectal septum at 3–7 weeks’ gestation.5 The caudal aspect of the urorectal septum forms the perineal body. The urogenital triangle lies anterior to the perineal body and the anal triangle posterior. The cranial portion of the urogenital sinus forms the bladder, the middle portion helps form anterior pelvic structures and the inferior portion the urogenital membrane. The common excretory duct is incorporated into the inferior portion of the bladder and proximal urethra during development. The ureteric ostia migrate laterally and cranially during bladder development while the distal portion of the metanephric duct remains medial and continues to communicate with the urethra forming ejaculatory ducts and seminal vesicles. Tissues between the developing distal ureter and the ejaculatory duct orifice form the superficial muscular layer of the trigone. The deep muscular layer of the trigone is contiguous with the detrusor muscle of the bladder. The genital ridges fuse to form the urethra in males or remain separate to form major and minor labia in females. The urachus develops from the urogenital diaphragm and connects the dome of bladder with the fetal allantoic duct within the space of Retzius anterior to the peritoneum and posterior to the abdominal wall musculature. Normally the urachus fibroses during the second trimester and forms the median umbilical ligament. The upper urinary tract consists of paired kidneys and ureters, while the bladder and urethra constitute the lower urinary tract. The developing kidney gradually rotates medially and undergoes cranial migration relative to the spine due to lengthening of the lumbar and sacral spine during development, so that the normal final position is in the upper lumbar region. Arterial supply during development is from progressively more cranial aortic branches beginning initially at the lateral sacral artery. Final arterial supply is from the aorta at the level of the second lumbar vertebral body, by which time arteries that arose earlier in development should have involuted.6 Accessory unilateral renal arteries occur in approximately 30% of the population and bilaterally in 10%.7 The prevalence is higher when abnormal renal ascent occurs.6 The left renal vein generally drains anterior to the aorta into the IVC. Involution anomalies of the embryonic circumaortic venous ring lead to retroaortic and circumaortic left renal drainages which are not normally associated with other renal deformities.6 In this configuration, the ipsilateral adrenal and gonadal veins drain into the anterior and posterior renal veins, respectively. The renal veins lie anterior to the renal arteries, which lie anterior to the renal pelvices. The kidneys are contained within the perirenal space, one of four retroperitoneal spaces which also include the anterior and posterior pararenal spaces, and the retroperitoneal vascular compartment. The perirenal space is bounded by a thin layer of connective tissue called Gerota’s fascia (sometimes subdivided into a posterior fascia of Zuckerkandl and lateroconal fascia). Superiorly Gerota’s fascia is closely related to the adrenal glands; it also envelops the renal pedicle consisting of the renal artery, vein, lymphatics and the urinary collecting system. The perirenal space contains thin septations called Kumin’s septa that may thicken in response to renal disease, an appearance termed perinephric stranding. The perirenal space communicates with the bare area of the liver or spleen superiorly, the periureteric tissues inferiorly and the renal sinus fat medially. The perirenal spaces may communicate across the midline and with the retroperitoneal vascular space. The pararenal spaces communicate caudally and with the extraperitoneal spaces, including the prevesical space. The renal collecting system consists of 10–14 concave-shaped minor calyces bounded laterally by forniceal angles. The minor calyces coalesce to form upper, mid and lower major calyces, or infundibula, and then the renal pelvis is either enveloped entirely in the renal sinus, or partially outside the kidney, termed an extrarenal pelvis. Exuberant adipose proliferation termed renal sinus lipomatosis may occur in the renal sinus with increasing age, renal atrophy or steroid administration.4 The normal kidney contains 14 lobes, each consisting of a calyx, collecting duct and cortex. The external contour of the kidney demonstrates lobulation at the intersection of lobes, evident up to five years of age as the kidneys mature and cells multiply. Beyond this age, external lobulation disappears and glomeruli may respond to injury such as partial or complete nephrectomy by hypertrophy. The normal kidney should be 12–14 cm in length and there should be less than 1cm difference in lengths between the kidneys. Renal length tends to be underestimated by ultrasound and overestimated by intravenous urography. The ureters have three normal constrictions at points of temporary peristaltic arrest; at the pelvic inlet, crossing the iliac vessels, and at the ureteropelvic junctions. The normal ureter courses along the anterior psoas muscle within 1cm of the lateral margin of the vertebral transverse processes and outside the line of the pedicles to the level of L3 on an abdominal radiograph, sometimes with a short horizontal course as it crosses the lateral edge of the psoas at L3 (Fig. 35-1). The transverse distance between the ureters at this level should normally be greater than 5 cm. The bladder has a superior surface with a peritoneal reflection, two inferolateral surfaces, a neck, which contains the ureteric ostia, a base which contains the trigone, and continues into the urethra from the apical region as the medial umbilical ligament. The trigone is bounded by the ureteric orifices posterolaterally and the urethral orifice anteroinferiorly. The muscular layer of the bladder is known as the detrusor muscle and the bladder is lined by transitional cell epithelial. The anterior division of the internal iliac artery supplies the bladder via the superior, middle, and inferior vesicle arteries. Many collateral arteries exist, including the obturator and inferior gluteal arteries. Venous drainage from the bladder is into the internal iliac vein, and lymphatic drainage occurs into internal, external, and common iliac nodal basins. The male urethra consists of prostatic, membranous, bulbous, and penile divisions. The prostatic portion is lined by transitional cell epithelium, and the remainder by columnar epithelium. The prostatic and membranous portions constitute the posterior urethra. The prostatic portion has a posterior indentation as it traverses the transitional zone of the prostate called the verumontanum, which serves as a landmark for prostatic gland drainage lateral to this structure. The prostatic utricle is a vestigial remnant of the Müllerian duct located just inferior to the verumontanum and is the site of ejaculatory duct drainage. The membranous portion of the urethra has a thick outer circular rim of muscle, which forms the external urethral sphincter. This is the narrowest and shortest portion of the urethra, and is followed by the widest urethral segment, the bulbous urethra within the corpus spongiosum of the penis, from the level of the urogenital diaphragm which separates the posterior and anterior urethra (Fig. 35-2). The paired Cowper’s glands drain into the proximal bulbous portion. The remainder of the anterior urethra is termed the penile portion from the level of the penoscrotal junction. The female urethra is widest at its origin and narrowest at the meatus adjacent to the external vagina. Skene’s glands drain along the course of the urethra. The female urethra is approximately 4 cm in length and the external urethral sphincter is in the region of the mid urethra. Renal abnormalities may be subclassified into those of number, position, fusion, vasculature, and ureteropelvic junction anomalies.8 Failure of the ureteric bud to join the metanephric blastema causes renal agenesis. This is observed in 1 in 1000 live births and is associated with absence of the ipsilateral ureter or the presence of a ureteric stump.6 Absent ipsilateral epididymis, vas deferens, seminal vesicle or presence of a seminal vesicle cyst is observed in up to 70% of patients with renal agenesis.1 Interestingly, anomalies of the urinary tract are almost twice as common among men compared with women; however, congenital anomalies of the genital tract are much more common in women.1 Woman patients with renal agenesis have a 70% incidence of Müllerian anomalies such as vaginal and uterine agenesis, or unicornuate uterus, which are part of Mayer–Rokitansky–Küster–Hauser syndrome. Absent ipsilateral adrenal gland is observed in 10%. Agenesis of the left kidney can be recognised on an abdominal radiograph if the splenic flexure is located medial to the lesser curve of stomach. Bilateral renal agenesis results in Potter’s syndrome (1 in 3000 live births) consisting of oligohydramnios, pulmonary hypoplasia and facial anomalies such as low set ears, broad flat nose and prominent infraorbital skin folds. Supernumerary kidney on the other hand refers to the presence of an additional kidney. A supernumerary kidney is uncommon but normally occurs on the left, probably due to duplication of the ureteric bud. The supernumerary kidney is located caudal to the normal kidney drained by either a separate ureter or a branch of a bifid ureter. An abnormal amount of renal rotation around its long axis can position the ureteropelvic junction anterior, or less commonly, posterior to the kidney. Abnormal caudocranial renal ascent during development results in an ectopic renal location (Fig. 35-3). An ectopic kidney is more commonly inferior to the normal anatomical position, or rarely, in the upper abdomen where it is referred to as a thoracic kidney. Ectopic kidneys can be located in the pelvis, in the iliac fossa or can cross the midline. There is an increased incidence of renal anomalies in both the ectopic kidney and its contralateral counterpart. A pancake kidney is produced when bilateral pelvic kidneys fuse (Fig. 35-4). Pelvic kidneys are more prone to decreased function, ureteropelvic junction obstruction, vesicoureteric reflux, stone formation and trauma (Fig. 35-5). Fusion anomalies of several types and severity occur in the kidneys. A horseshoe kidney deformity consists of medial facing lower renal poles connected by an isthmus containing parenchyma or a relatively avascular fibrous tissue, which rotates the renal axis (Fig. 35-6). This anomaly occurs following metanephric contact and fusion during development. The incidence of horseshoe kidney is approximately 1 in 400 live births and has a 2-to-1 male-to-female preponderance.4 The isthmus lies anterior to the inferior vena cava and aorta but posterior to the inferior mesenteric artery. As a result, renal assent is arrested at this level. Horseshoe kidney is associated with a 30% incidence of ureteropelvic junction obstruction, stone formation in 30%, and 10% chance of ureteric duplication. There is an increased incidence of genital, cardiovascular and anorectal abnormalities. Patients with horseshoe kidneys also have increased risk of renal tumours including Wilms’ tumour, traumatic injury or infection. Occasionally, one kidney crosses the midline and fuses with the contralateral kidney, a configuration known as crossed fused ectopia (Fig. 35-7). Unlike horseshoe kidney, both kidneys lie on one side of the spine (Fig. 35-8). Generally, the ureters drain in an orthopic manner into the bladder when crossed fused ectopia exists. This fusion pattern is more common in men and the left kidney is more commonly ectopically located (Fig. 35-9). Overall, abnormalities of the renal vasculature occur in almost 25% of the population. Ectopic kidneys have a higher incidence of anomalous blood supply. Renal artery duplication is the most common vascular abnormality. The accessory artery normally supplies the lower pole renal territory. Accessory veins are much less common than accessory arteries. Vessels crossing the ureteropelvic junction can cause complete segmental renal obstruction, which can simulate duplication (pseudodupliction). Anomalous vessels generally cause few symptoms; however, inadvertent injury during open aortic and renal surgery, or ureteroscopic procedures can occur. The tissues anterior to the ureteropelvic junction, in particular, should be examined carefully on pre-procedure imaging, since anomalous vessels traverse anterior more frequently than posterior to the ureteropelvic junction. Congenital ureteric anomalies can be generally subdivided into those of ureteric number, direction, diameter and filling defects.8 The most common ureteric anomaly is that of ureteric number, which was observed in 0.7% of autopsies in one series.9 The renal parenchyma may be divided into upper and lower components by a septum of Bertin. This anomaly is associated with a bifid collecting system consisting of two pelvices joined proximal to the ureteropelvic junction (Fig. 35-10). The upper calyces drain into the upper pelvis, while the mid and lower calyces drain into the lower pelvis. The ureters may also join distal to the ureteropelvic junction (Fig. 35-11) with a Y-configuration. This is normally asymptomatic unless urine refluxes from one ureter up the other causing ureteroureteric reflux, also termed yo-yo reflux, which should be considered if one ureteric segment is disproportionately enlarged.10 This anomaly can precipitate recurrent infections and obstruction of a ureteric segment. When there is complete duplication of the renal collecting system, the ureter draining the upper moiety tends to insert ectopically below and medial to the normal ureteric orifice or sometimes outside the bladder (extravesical). The ureter draining the upper moiety tends to drain ectopically due to delayed separation of the ureteric bud from its parent Wolffian duct, which migrates in a caudal direction during development.11 For this reason it is suggested that if the upper urinary tract is normal, the ureter is likely to have an orthopic insertion; however, a dysplastic or malfunctioning upper tract is likely associated with an ectopic insertion. The ectopic ureter often has a strictured distal element which causes proximal dilatation in the bladder wall and ureterocele formation in 75% of affected patients and is eight times more common in women than men (Fig. 35-12).10 A ureterocele contains layers of ureteric and bladder epithelium which are optimally appreciated on early imaging on a voiding cystourethrogram (VCUG) but can be obscured by contrast medium, effaced or everted similar to a diverticulum when the bladder is full (Fig. 35-13).12 A cobra-neck appearance is observed at intravenous urography (IVU) consisting of a dilated contrast-filled ureter surrounded by thin rim of ureteric wall and bladder mucosa measuring less than 2 mm in diameter (Fig. 35-14). Ectopic ureteroceles often project into the bladder and are associated with recurrent infection, stone formation, complete ipsilateral or bilateral ureteric obstruction. Orthopic ureteric insertion in the setting of ureteric duplication is generally asymptomatic unless larger than 2 cm. An ectopic ureter with extravesical insertion below the bladder neck, into the urethra or vagina, can cause urinary incontinence, infection and ureteric obstruction from fibrotic stricture formation. The ectopic ureter generally inserts above the external sphincter in men, thereby inhibiting incontinence but in about 50% of women this does not occur and patients present with dribbling incontinence but are able to void normally. The ureter draining the lower moiety of a duplicated collecting system inserts orthopically but tends to reflux. In fact, reflux is the most common association of ureteric duplication. Lower pole hydronephrosis in the setting of duplication is mainly due to reflux rather than an obstructing ureterocele or stricture. In duplicated systems the dilated upper pole ureter can impinge and displace the lower pole ureter, which can be appreciated on voiding cystourethrogram. Conformity of a duplicated collecting system with this pattern of ureteric insertion is known as the Weigert–Meyer Rule.10 Approximately one-third of patients with complete ureteric duplication have an additional congenital anomaly such as horseshoe kidney and multicystic dysplastic kidney. Anomalies of ureteric course generally occur due to extrinsic processes, which push or pull complete or segmental portions of the ureter in medial or lateral directions. Potential causes of medial ureteric deviation include retrocaval ureter (Fig. 35-15), laterally based renal, retroperitoneal, lymph node, central pelvic or psoas masses, pelvic lipomatosis, vascular aneurysms or haematoma, retroperitoneal fibrosis and surgical resection of pelvic organs such as the entire rectum. Lateral ureteric deviation is caused by retroperitoneal and central pelvic masses such as lymphadenopathy (testicular metastases), aortic aneurysm, psoas enlargement (young men) and abnormal renal position (malrotated or horseshoe kidney). Cross-sectional imaging is usually required to confirm aetiology. Acute medial deviation of the right ureter medial to corresponding vertebral pedicles on an abdominal radiograph is virtually diagnostic of retrocaval ureter. Pelvic lipomatosis pushes the distal ureters medially. This condition is more common in African-American men and can induce bilateral hydronephrosis. A characteristic pear-shaped bladder may be observed and cystitis glandularis of the bladder is an associated premalignant condition. Retroperitoneal fibrosis pulls one or both ureters medially in about half of cases. Psoas muscle hypertrophy can be recognised as the cause of lateral ureteric deviation if the lateral psoas border exceeds 8 cm from the lateral edge of the corresponding vertebral body at the level of the iliac crest. Other congenital ureteric anomalies of note include ureteric stricture, megaureter and vesicoureteric reflux. Ureteric strictures are the most common of these anomalies. The ureteropelvic junction, in particular, and the ureterovesical junction to a lesser extent, are predisposed to stricture formation. Ischaemia during development is thought to incite fibrosis and stricture formation in these regions. There is consequent dilatation of the proximal ureter and possibly the renal pelvis, which may be detected by screening or as an incidental finding in asymptomatic patients, or associated with flank pain, recurrent infection, haematuria or stone formation, anywhere from in utero to adulthood (Fig. 35-16). Ureteric strictures may also be caused by a vessel crossing from medial to lateral with a convex lateral margin in about 1 in 20 cases of ureteropelvic junction obstruction (Fig. 35-17). Ureteric diameter exceeding 7 mm is termed megaureter.13 Megaureter may be a congenital phenomenon or secondary to a process in the bladder or urethra (Fig. 35-18). Primary megaureter is due to deficient ureteric musculature, which limits peristalsis and leads to upstream dilatation. Three forms are described: obstructing primary megaureter, refluxing primary megaureter, and non-obstructing non-refluxing primary megaureter. Primary refluxing megaureter is due to inadequate one-way valve mechanism at the vesicoureteric junction. In non-obstructing non-refluxing primary megaureter the calyces should maintain a crisp outline and not be blunted as with primary obstructing megaureter and there should be normal excretion (Fig. 35-19). Three-quarters of cases are unilateral; there is left-sided a male predominance. Vesicoureteric reflux is caused by maldevelopment of the distal ureter characterised by a shortened intramucosal bladder tract with a deficient valve-like mechanism. Reflux increases the risks of renal infection, scarring and reflux nephropathy. Vesicoureteric reflux represents the most common cause of antenatal hydronephrosis, responsible for 40% of cases.14 Grading is between 1 and 5 according to the International Reflux Study Committee as determined by the most severe degree of reflux at voiding cystourethrography: reflux into the ureter only (I), to the renal pelvis without calyceal dilatation (II), with mild or moderate ureteric and calyceal dilatation but preserves fornices (III), and severe ureteric and calyceal dilatation with obliteration of forniceal angles but maintained greater calyceal papillary impressions (IV), and finally gross dilatation with effacement of the papillary impressions (V).15 The term cloaca is derived from the Latin term for sewer. Cloacal malformation is believed to occur when the urorectal septum fails to separate the cloaca and results in a shared orifice for the genital, urinary and lower gastrointestinal tracts in a phenotypic female. A urogenital sinus anomaly occurs at a later stage in development and results in a common drainage tract for the genital and urinary systems. Extrophy of the cloaca occurs in males and females when there is failed closure of the infra-umbilical anterior abdominal wall, creating a defect larger than that observed with bladder extrophy. Several anomalies of bladder development may occur, including agenesis, duplication, diverticula, prune-belly syndrome and anomalies of the cloaca. Agenesis of the bladder is very rare and its origin is uncertain, but since the hindgut often develops normally, maldevelopment of the anterior cloaca is usually responsible. The ureters insert ectopically in this situation and further urinary tract anomalies are common. Rarely, there may be duplication of the bladder and urethra created by a septum containing detrusor muscle. This occurs more commonly in a sagittal rather than in a coronal plane. This anomaly is associated with uni- or bilateral renal obstruction, single or duplicated urethras, genital or lower gastrointestinal tract duplication. Bladder diverticula in children in the absence of obstruction have an incidence of 1.7%.16 A diverticulum at the vesicoureteric junction is known as a Hutch diverticulum and is associated with vesicoureteric reflux (Fig. 35-20). The term bladder ear, on the other hand, refers to a diverticulum which descends into the internal inguinal ring (Fig. 35-21). Congenital diverticula are often asymptomatic; however, infection, stone formation and possibly cancer can occur, so surgical repair is sometimes necessary. Prune-belly syndrome, also known as Eagle–Barrett syndrome, is a rare constellation of findings consisting of absent rectus abdominis muscles with lax abdominal wall skin, bilateral undescended testes, dilated ureters and prostatic urethra and abnormal kidneys.2 Vesicoureteric reflux is a common association and affected neonates generally die early. The bladder wall is characteristically thickened and the bladder enlarged though not trabeculated, owing to increased mural connective tissue. Associated anomalies are common, including those of the musculoskeletal, cardiorespiratory or gastrointestinal systems. The urachus is often patent in the setting of prune-belly syndrome. Failure of the urachus to fibrose and obliterate causes a constellation of anomalies. Patent urachus presents with urine draining from the umbilicus at birth. Urachal cysts occur when the umbilical and vesical portions are occluded but a central segment remains patent. A urachal sinus forms when there is a blind-ending tract within the urachus contiguous with the umbilical opening, or a urachal diverticulum is observed when a segment of urachus remains in continuity with the bladder.17 Infection is the most common complication affecting urachal remnants. Adenocarcinoma of urachal remnant is a rare cancer that often produces mucin and may contain calcification. Prognosis is typically worse compared with bladder malignancies. Posterior urethral valves are the most common malformation of the urethra. The valve is created from a remnant of the Wolffian duct and consists of a raised mucosal fold which courses obliquely from the verumontanum to the inferior aspect of the prostatic urethra (Fig. 35-22). Diagnosis is often made after bilateral hydronephrosis is detected. Bladder outflow obstruction raises intravesical pressure, causes bladder wall thickening, trabeculation, and vesicoureteric reflux in approximately half of affected patients (Fig. 35-23
The Urinary Tract
Overview of Anatomy, Techniques and Radiation Issues
Introduction
Anatomy of the Urinary Tract
Embryology
Normal Urinary Tract Anatomy
Congenital Abnormalities of the Urinary Tract
Renal
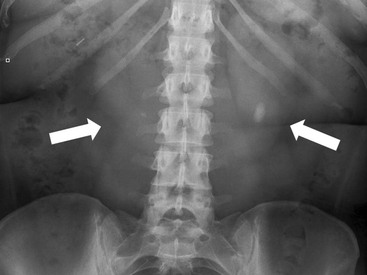
Ureter and Pelvis
Bladder and Urethra
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Urinary Tract
Chapter 35