Algorithm 21.1 Decision tree detailing the evaluation of collecting system dilatation
UPJ Obstruction
Obstruction of the ureteropelvic junction (UPJ) is the most common congenital cause of hydronephrosis. The pathogenesis of UPJ obstruction is not fully understood, but congenital UPJ obstruction is most likely an intrinsic muscular deficiency, resulting in failed relaxation at the UPJ [2], somewhat analogous to achalasia of the esophagus.
The so-called crossing vessels have also been implicated in UPJ obstruction. In this case, when a patient is found to have UPJ obstruction, investigation may reveal a retroperitoneal artery or vein crossing anterior to the ureteropelvic junction, producing at least a visual sensation in some cases that the crossing vessel is actually causing the obstruction by extrinsic compression (Figs. 21.1, 21.2, and 21.3). However, these “crossing vessels” are also found in normal individuals and their functional significance in patients with UPJ obstruction is therefore controversial [2]. Nevertheless, crossing vessels are important to define in patients with UPJ obstruction in whom surgical correction (endopyelotomy) is contemplated, given the increased risk of retroperitoneal hemorrhage [3].
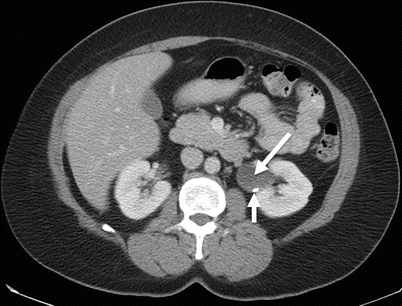
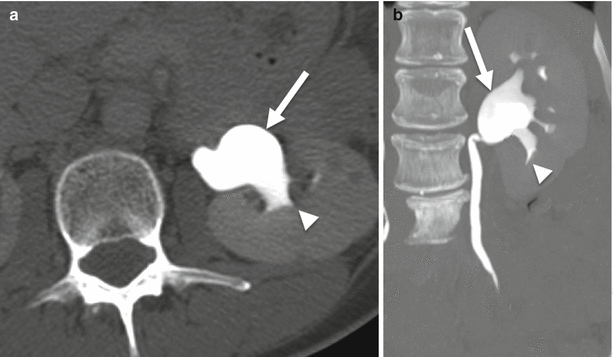
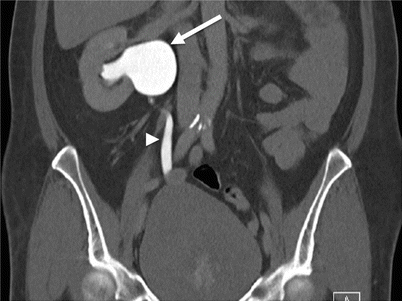

Fig. 21.1
Extrarenal pelvis. Nephrographic phase CECT. Dilated left extrarenal pelvis (long arrow) containing a small, nonobstructing calculus (short arrow)

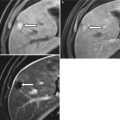
Fig. 21.2
Extrarenal pelvis. Axial (a) and coronal maximum intensity projection (b) excretory phase CT urogram. Dilated renal pelvis (arrows) but sharp renal calyces (arrowheads) and normal ureter

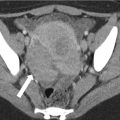
Fig. 21.3
Ureteropelvic junction obstruction. Coronal excretory phase CECT. Markedly dilated right renal pelvis (arrow) with normal caliber proximal ureter (arrowhead)
Extrarenal Pelvis
Variations in position and shape of the renal pelvis are common and can mimic obstructive uropathy. When situated outside the renal sinus, the renal pelvis is unconfined by solid renal parenchyma and consequently may appear bulbous and dilated, even when unobstructed. Extrarenal pelves are occasionally difficult to distinguish from mild cases of ureteropelvic junction obstruction since the ureter is normal caliber in both conditions (Figs. 21.4 and 21.5). However, identification of normal decompressed calyces favors a diagnosis of extrarenal pelvis. When the diagnosis remains in doubt, especially in pediatric patients, functional imaging using radionuclide renography is often utilized to help exclude UPJ obstruction.
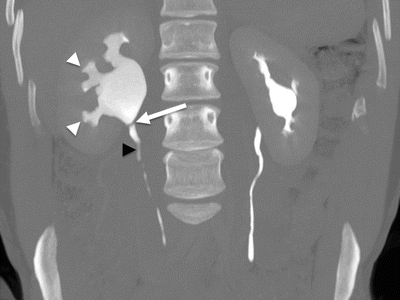
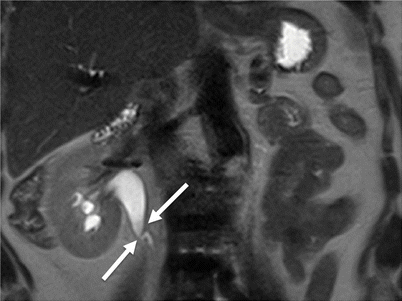

Fig. 21.4
Ureteropelvic junction obstruction. Coronal MIP CT urogram. Marked right calyceal dilation and blunting (white arrowheads) and dilated right renal pelvis, with rapid tapering at the ureteropelvic junction (white arrow) to normal caliber ureter (black arrowhead). Normal left renal collecting system and ureter

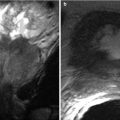
Fig. 21.5
Ureteropelvic junction obstruction. Coronal T2-weighted MR. Right pelvicaliectasis with rapid tapering at the ureteropelvic junction (arrows) to normal caliber ureter
Congenital Megacalycosis
Congenital megacalycosis (CM) is a very rare developmental anomaly caused by hypoplasia or failed development of the renal medullary pyramids [4]. The condition is characterized by (1) an increased number of (2) oversized renal calyces (Fig. 21.6). This entity can be distinguished from hydronephrosis and congenital UPJ obstruction in that the size of the renal pelvis and ureter in CM is normal.
Complications of CM include formation of renal calculi and recurrent urinary tract infections [5], either of which may precipitate discovery of the condition.
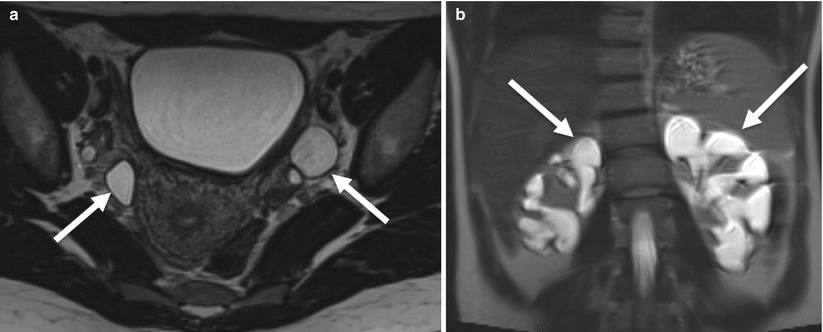

Fig. 21.6
Congenital megaureter with megacalyces. Axial (a) and coronal (b) T2-weighted MR. (a) Bilateral markedly dilated distal ureters (arrows). (b) Bilateral markedly dilated renal calyces (arrows)
Calyceal Diverticulum
A calyceal diverticulum is a cyst-like lesion of the renal parenchyma but is differentiated from a true renal cyst by the presence of a communication with the renal collecting system. Most calyceal diverticula are likely congenital in etiology, while a minority may be sequelae of prior infection or stone disease (Fig. 21.7). Most commonly, calyceal diverticula are found at the upper pole of the kidney in relation to a minor calyx and typically rather small. Less common is a diverticulum found in the central interpolar region of the kidney in relation to a major calyx or the renal pelvis. These tend to be quite large [6].
Like any site of urinary stasis, calyceal diverticula can harbor infection and stone formation. Calyceal diverticula are easily distinguished from renal cysts by excretory phase imaging: calcyceal diverticula opacify with contrast, whereas renal cysts do not. Even a shallow contrast pool within a cystic structure is considered pathognomonic.
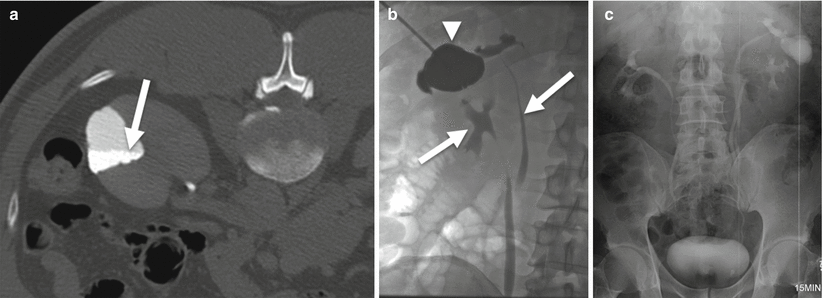

Fig. 21.7
Calyceal Diverticulum. (a) Axial prone excretory phase CT urogram. Contrast-opacified, triangular parenchymal defect. Note fluid-fluid level resulting from layering contrast material within stagnant urine (arrow). (b) Antegrade nephrostagram. Direct contrast injection of diverticulum (arrowhead) with subsequent filling of a duplicated collecting system (arrows). (c) Intravenous pyelogram
Obstructive Uropathy
Urinary obstruction is one of the most common urologic entities encountered in clinical practice and radiology. Obstruction is seen in all age groups and the causes are numerous, both congenital and acquired and benign and malignant. Among these, the vast majority of cases are acquired benign conditions—mostly urolithiasis.
Cases of chronic urinary obstruction may go unnoticed by the patient for many years, even when severe. When given time to accommodate, the renal pelvis and ureter may balloon to massive size without sequelae of infection, inflammation, or rupture. However, chronic urinary stasis does favor stone formation and infection. The natural course of unrecognized or untreated hydronephrosis is renal parenchymal damage, progressing to renal atrophy and loss of renal function.
On the other hand, acute obstruction, even when causing only mild hydronephrosis, can present with severe symptoms of renal colic and flank pain but little (if any) measurable change in renal function. Acute obstruction may be complicated by forniceal rupture with retroperitoneal leakage of urine (Figs. 21.8 and 21.9).
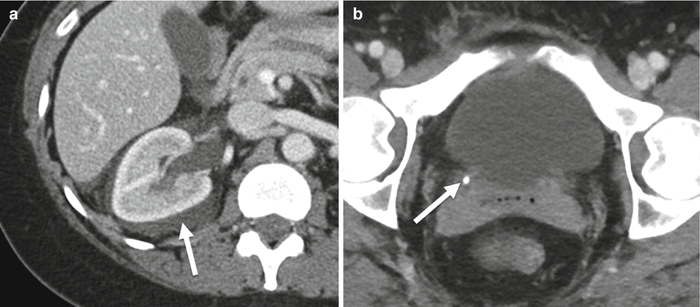
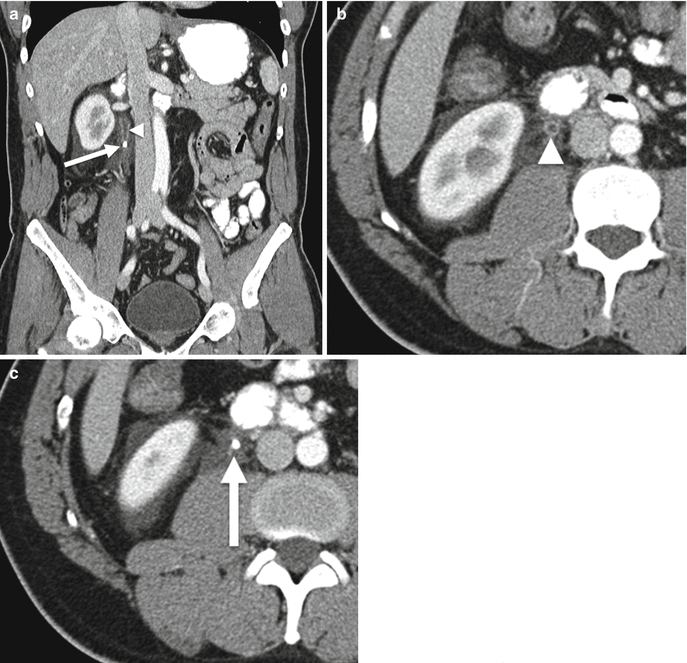

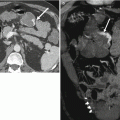
Fig. 21.8
Forniceal rupture. Axial corticomedullary phase CECT. (a) Right perinephric and peripelvic fluid (arrow) but relatively decompressed renal collecting system, signifying forniceal rupture secondary to (b) obstructing calculus at the ureterovesicle junction (arrow)

Fig. 21.9
Forniceal rupture. Coronal (a) and axial (b, c) corticomedullary phase CECT. Obstructing stone within the right proximal ureter (arrows). Upstream urothelial enhancement consistent with reactive inflammation (arrowhead). Perinephric and periureteral fluid suggestive of forniceal rupture
Various causes can lead to the visualization of filling defect within the urinary tract. Algorithm 21.2 illustrates common etiologies for filling defects within the urinary tract.
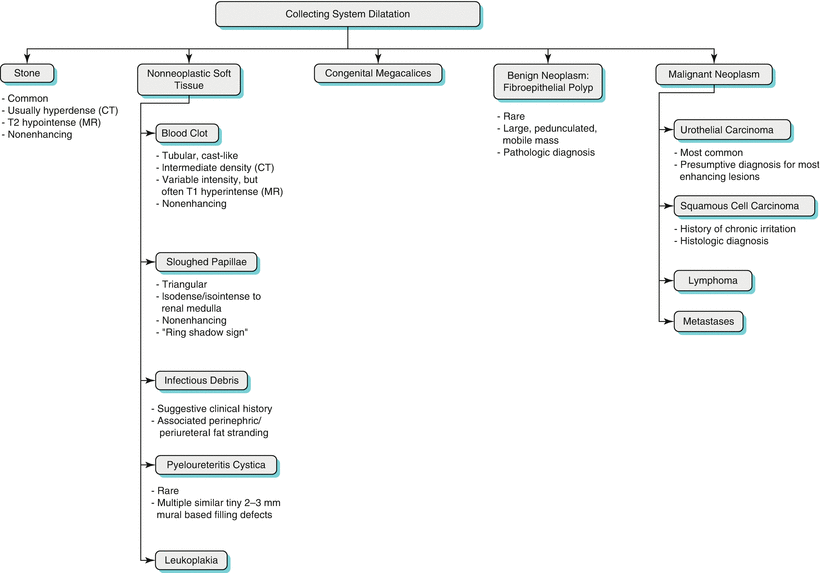

Algorithm 21.2 Systematic approach to determining the etiology of filling defects in the upper urinary tract
Urolithiasis
Traditional radiography remains relatively sensitive (up to 85 %) in detection of urinary stones, because most stones contain calcium. However, identification of stones on radiographs is often hampered in patients of large body habitus and confounded in patients with non-urinary calcifications, such as vascular phleboliths.
On the other hand, by adding the ability to identify non-calcium stones (uric acid, xanthine, and cystine), MDCT raises the sensitivity for stone detection up to 98 % [7]. Only pure matrix stones and indinavir stones are likely to be missed at noncontrast CT because they are of soft tissue attention, although secondary changes of obstruction may imply their presence anyway [7].
Most calculi within the renal collecting system are too small to cause obstruction, and many are often discovered incidentally on CT imaging performed for other indications. Nevertheless, careful attention is warranted to the “incidental” stone, as the findings of local obstruction of a single urinary calyx may be quite subtle. Comparison with adjacent normal calyces or with the contralateral normal kidney may help confirm localized caliectasis. Besides dilatation, secondary imaging findings of urinary obstruction mimic those of infection and inflammation: stranding of the periureteral and perirenal fat.
Urinary calculi naturally lodge at the anatomic points of narrowing along the urinary stream, namely, (from proximal to distal) the calyceal-infundibular junction, the ureteropelvic junction, the ureterovesicle junction, and the bladder neck.
Sloughed Papillae
A sloughed papilla refers to the detached papillary tip that occurs after ischemic necrosis. The renal medulla and especially the papillary tips are vulnerable to ischemia from any cause, but common causes include diabetes mellitus, analgesic/NSAID abuse, renal vein thrombosis, sickle cell disease, and hypotensive shock. Once detached, sloughed papillae may be seen as noncalcified and nonenhancing filling defects in the renal pelvis. On excretory phase imaging, a triangular filling defect may be seen surrounded by a thin rim of contrast material, referred to as the “ring-shadow sign.” Like any mobile filling defect, sloughed papillae can migrate into the ureter, resulting in obstruction with urinary colic, or pass distally without complication or symptoms. Old sloughed papillae may calcify, thus representing a rare cause of urinary calculi.
Sloughed papillae are occasionally distinguished from other soft tissue filling defects by their classic triangular shape. Unlike urothelial carcinoma, sloughed papillae do not enhance.
Blood Clot
Renal hemorrhage with clot retention in the ureter is an important but uncommon cause of urinary obstruction. Typically, renal hemorrhage in these cases is caused by trauma, including accidental blunt trauma, such as a motor vehicle collision, as well as iatrogenic trauma, such as hematuria following percutaneous renal biopsy or nephrostomy. Bleeding caused by a proximal urothelial carcinoma may also result in obstructive ureteral blood clots.
Ureteral blood clots can be distinguished from other ureteral filling defects by certain features. Blood clots typically form elongated or “tubular” filling defects, producing a “finger-in-glove”-like appearance when surrounded by excreted contrast media. Blood clots may appear of various density based on age and concentration of blood products but do not show enhancement following contrast administration.
Pyeloureteritis Cystica
Pyeloureteritis cystica is a benign condition characterized by subepithelial cysts that elevate the mucosal lining of the renal pelvis and/or ureter [8]. The condition is most commonly found in the proximal ureter (ureteritis cystica) but may also involve the renal pelvis (pyelitis cystica). As its name implies, pyeloureteritis cystica is thought to be related to chronic infection or inflammation, as seen with chronic urinary tract infection or chronic stone irritation. In and of itself, pyeloureteritis cystica is asymptomatic and considered an incidental imaging finding, though the causative process may be symptomatic.
Pyeloureteritis cystica is characterized on imaging by multiple small (2–5 mm), round, smooth-walled, eccentric filling defects protruding from the wall of the renal pelvis or ureter (Fig. 21.10). Although excretory phase imaging may show narrowing of the involved lumen, frank obstruction by submucosal cysts is very rare. The condition is usually stable over time and imaging follow-up is not routinely recommended. Pyeloureteritis cystica is not considered a premalignant condition, though it is controversial whether patients are at increased risk for development of urothelial carcinoma [8].
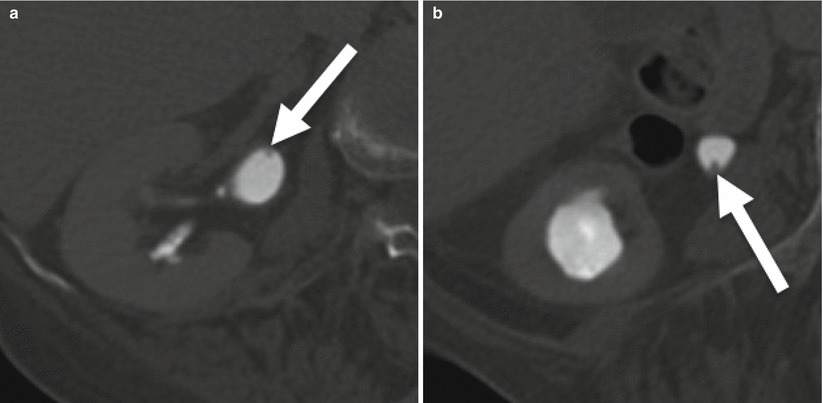
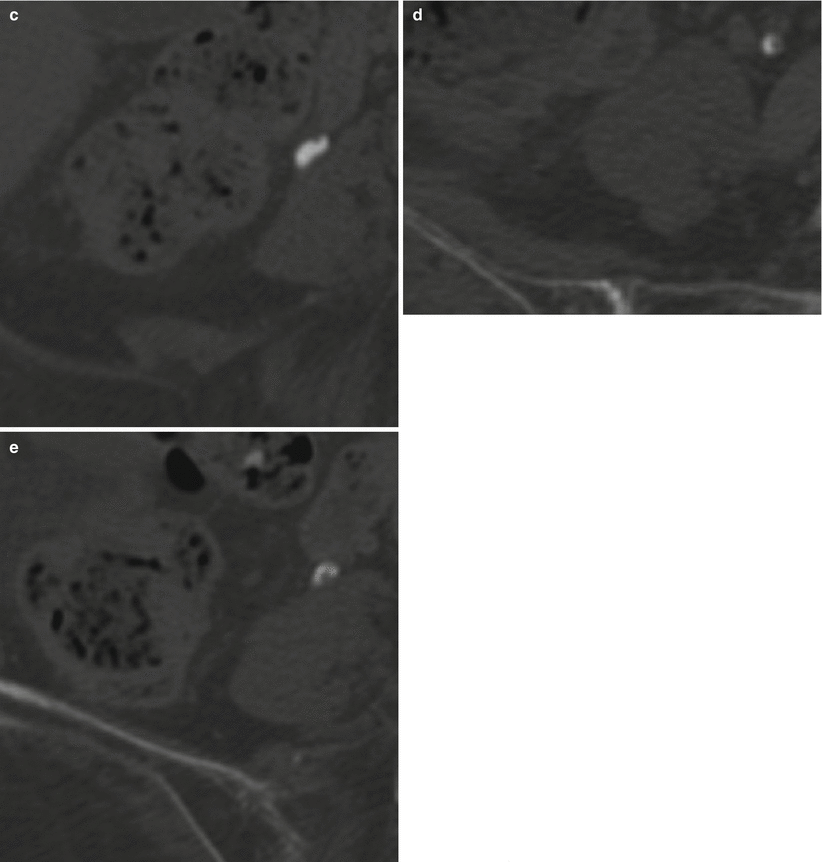


Fig. 21.10
Ureteritis cystica. Axial excretory phase CT urogram at the level of the renal pelvic (a) and progressing down the ureter (b–e). Multiple tiny (2–3 mm) filling defects arising from the walls of the right renal pelvis and ureter (arrows)
Leukoplakia and Malakoplakia
Leukoplakia is an uncommon lesion of the urothelium resulting from squamous metaplasia in the setting of chronic inflammation. Leukoplakia is characterized by plaque-like mural based filling defects which may be indistinguishable from urothelial carcinoma. Unlike pyeloureteritis cystica, leukoplakia is considered a premalignant lesion along the spectrum of development of squamous cell carcinoma. Indeed, when leukoplakia is found at one site in the urinary tract, a synchronous squamous cell carcinoma is often present.
Malakoplakia is another cause of a plaque-like filling defect in the upper urinary tract. However, malakoplakia represents a granulomatous reaction to chronic inflammation and is not considered a premalignant lesion [9]. Nevertheless, imaging differentiation of leukoplakia and malakoplakia from urothelial carcinoma is virtually impossible and tissue sampling is typically recommended.
Benign Neoplasms: Fibroepithelial Polyp
Most ureteral neoplasms are malignant and epithelial in origin, i.e., urothelial carcinoma. Conversely, a fibroepithelial polyp is of mesodermal origin and is the most common benign neoplasm of the ureter, yet fewer than 200 cases have been reported in the literature [10]. Fibroepithelial polyps are more common in adults ranging 20–40 years of age, but have also been found in children. They are usually solitary and most commonly found in the proximal ureter [11]. Excretory imaging demonstrates a smooth, slender, elongated filling defect ranging in size from 1 to 5 cm, though occasionally longer than 10 cm [10]. Because of their usual pedunculated attachment, they can appear as mobile ureteral masses and result in intermittent obstructive uropathy. Nevertheless, hematuria is a more common presentation than renal colic.
Malignant Neoplasm
Compared to tumors of the kidney and bladder, primary neoplasms of the renal pelvis and ureter are rare. In total, they account for only 5 % of all neoplasms of the urinary tract diagnosed each year [12]. Unfortunately, the vast majority of these tumors are malignant, with 85–90 % representing transitional cell carcinoma (TCC), now preferably termed urothelial carcinoma (UC) [12]. Squamous cell carcinoma accounts for an additional 10 % of upper urinary tract malignancies, usually resulting from squamous metaplasia occurring secondary to chronic irritation. Indeed, more than half of the patients with squamous cell carcinoma of the ureter also have urinary calculi [13]. Other histologies of upper urinary tract malignancy are extremely rare, including adenocarcinoma which accounts for less than 1 % of cases.
Certain chemical carcinogens—notably phenacetin—excreted in the urine are implicated in the pathogenesis of urothelial carcinoma and squamous cell carcinoma. This is thought to be due to the concentration and prolonged exposure that occurs along the urinary tract. Like urinary bladder cancer, males are twice more commonly affected than females [13].
By location, urothelial carcinoma of the upper urinary tract occurs most frequently in the extrarenal portion of the renal pelvis, followed by the calyceal-infundibular junction (Figs. 21.11 and 21.12). Ureteral UC is less common, accounting for only 25 % of cases. Approximately 60–75 % of cases of ureteral UC involve the distal third of the ureter [7, 8]. It is important to recognize that urothelial carcinoma has a propensity for multicentric disease: Synchronous bladder cancer is found in 2–4 % of patients with upper tract tumors, while metachronous bladder cancer will develop in 40 % of these patients [14]. Hence, detailed evaluation of the entire urinary tract is warranted at primary staging and surveillance imaging, especially during the first 24 months of surveillance when a majority of new lesions will develop [13].
Lesions in the renal pelvis (Figs. 21.13 and 21.14), like those in the urinary bladder, classically grow as a polypoid mass protruding into the lumen. When surrounded by contrast media on excretory phase CT or MRI or by T2 hyperintense urine on MRI, a classic polypoid filling defect is seen. However, even a small lesion involving a calyx or infundibulum may entirely plug the lumen such that no opacification occurs and no filling defect is depicted, resulting in the so-called phantom calyx [15]. Portal venous phase imaging can help in this instance, as any tumor filling the “phantom calyx” should enhance with contrast.
Due to its relatively narrow lumen, ureteral carcinomas are uncommonly seen as classic polypoid masses. Instead, these lesions tend to produce nonspecific soft tissue filling defects with or without surface irregularity [15]. The only sign in some cases may be a focal point of obstruction resulting in proximal hydroureteronephrosis. However, most patients present with hematuria before frank urinary tract obstruction. If these neoplasms form strictures, as they commonly will in the ureter, they may be circumferential or eccentric. They are often markedly irregular, a key differentiating feature from benign strictures. If smooth, clues to malignancy include non-tapering (“shouldered”) margins.
Multiphasic CT with CT urography is the current gold standard for imaging evaluation and characterization of urothelial carcinoma. However, MRI is gaining greater acceptance, particularly for its rendering of soft tissue contrast, in patients where use of iodinated contrast is contraindicated. CT urography with adequate ureteral distention and opacification allows detection of early localized disease, affording patients an increased likelihood of less extensive, curative, surgical options [15].
According to Browne et al., “TCC has lower signal intensity than the normally high-signal-intensity urine on T2-weighted images, permitting good demonstration of tumor in a dilated collecting system. However, TCC is nearly isointense to renal parenchyma on T1- and T2-weighted images, meaning that gadolinium based contrast agents are necessary for accurate assessment of tumor extent. Although TCC is a hypovascular tumor, moderate enhancement is seen with gadolinium contrast material, although not to the same degree as renal parenchyma.”
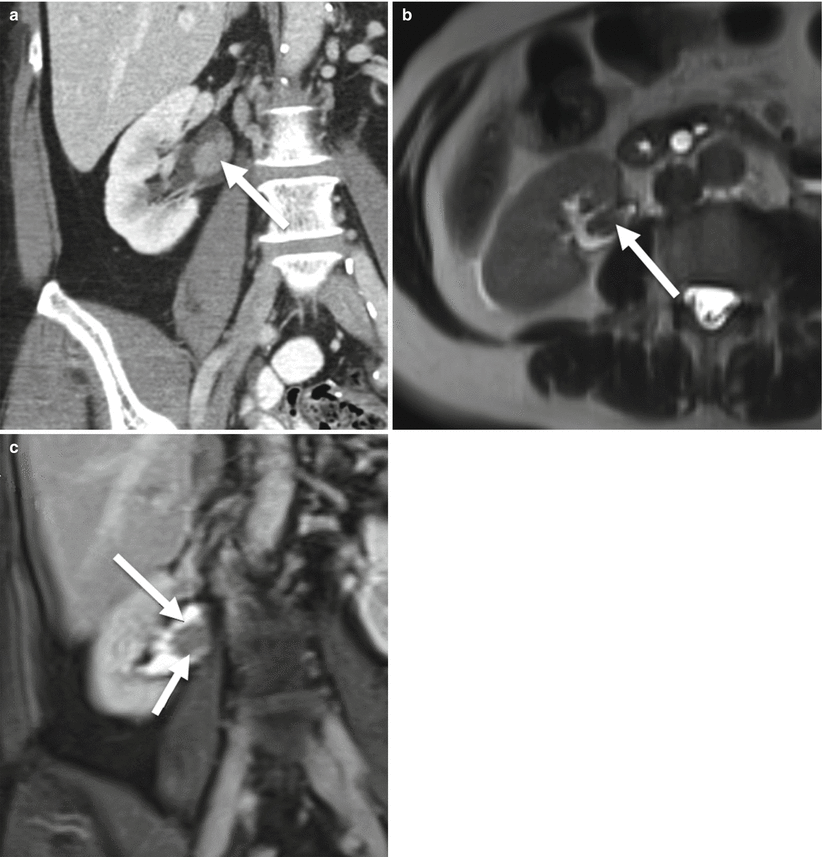
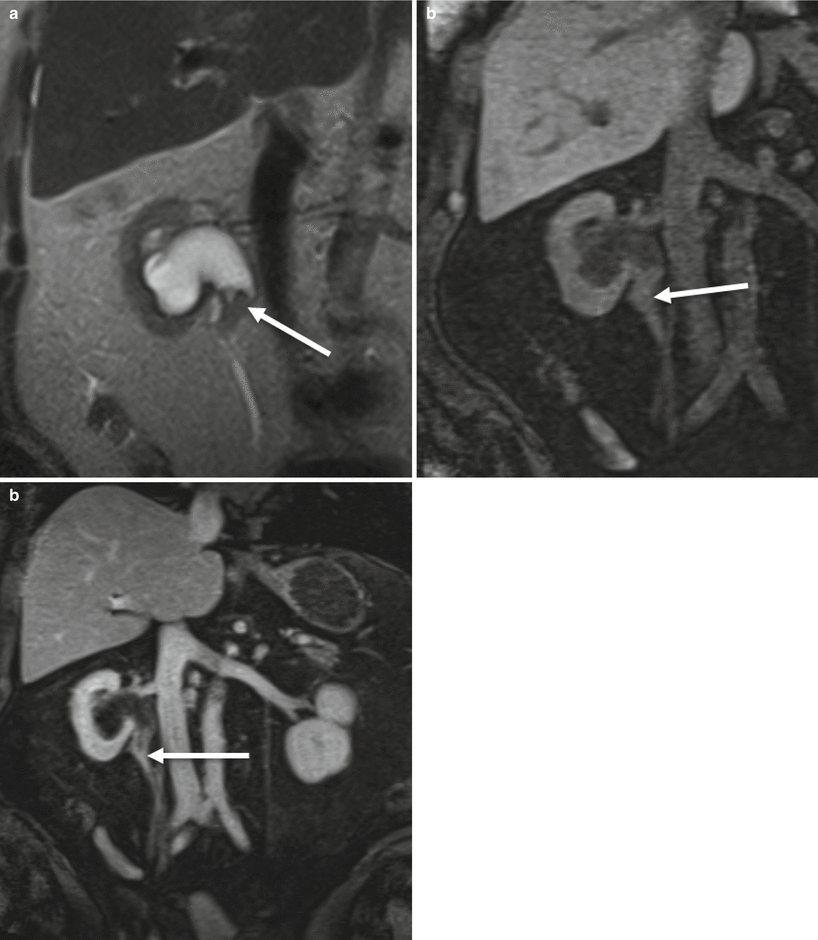
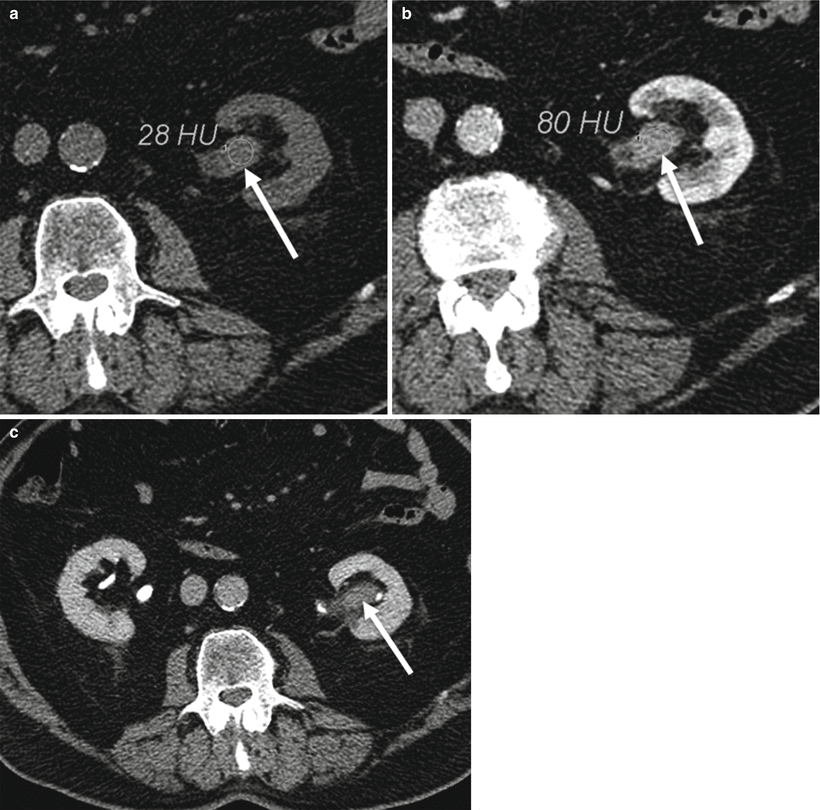
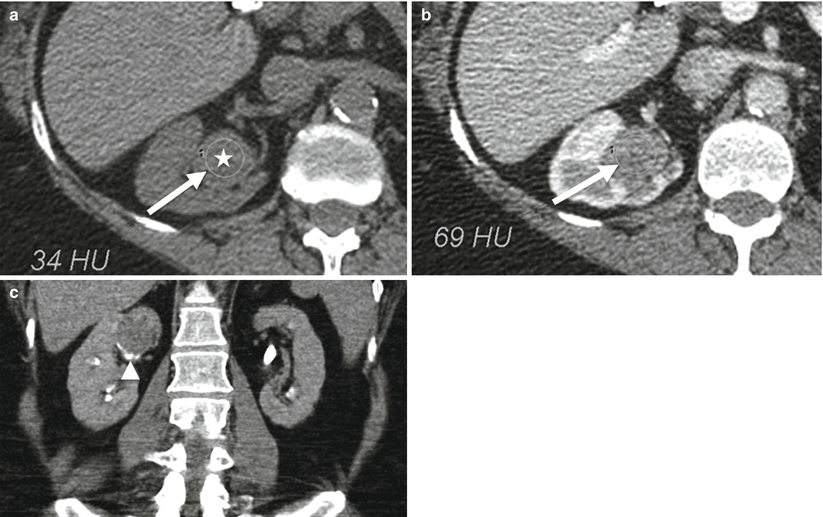

Fig. 21.11
Upper tract urothelial carcinoma. (a) Coronal nephrographic phase CECT. Enhancing soft tissue mass, right renal pelvis (arrow). (b) Axial T2-weighted MR. Intermediate to low signal filling defect, right renal pelvis (arrow). (c) Coronal T1+Gd. Enhancing soft tissue mass, right renal pelvis (long arrows). Histology showed urothelial carcinoma

Fig. 21.12
Upper tract urothelial carcinoma. (a) Coronal T2-weighted MR. UPJ pattern obstruction with intermediate signal filling defect at right UPJ (arrow). (b, c) Coronal pre- and post-contrast T1. Enhancing soft tissue mass at right UPJ (arrows). Histology showed high grade urothelial carcinoma

Fig. 21.13
Urothelial carcinoma. (a) Axial NECT. Vague abnormal soft tissue density in left renal pelvis (arrow). (b) Portal venous phase CECT. Enhancing soft tissue mass (arrow). Note the density measurements in Hounsfield units (HU) confirming enhancement (a, b). (c) Excretory phase CECT. Filling defect within left renal pelvis (arrow)

Fig. 21.14
Urothelial carcinoma. (a) Axial NECT with rounded soft tissue density distending an upper pole calyx (star). (b) Axial CECT confirms enhancement of this soft tissue mass. (c) Coronary excretory phase CT urogram. Round soft tissue filling defect within the right upper pole (arrow) with a claw of calyx at the inferior aspect of this lesion (arrowhead)
Various masses can surround the ureter. Algorithm 21.3 illustrates the most common periureteric masses.
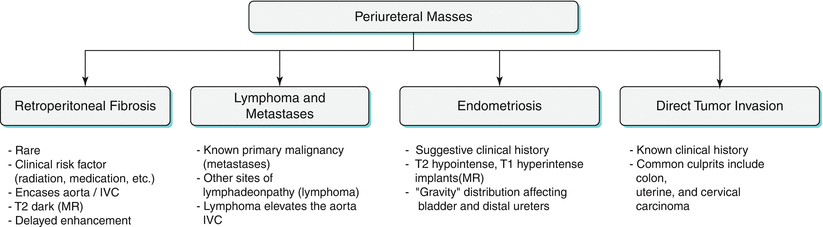

Algorithm 21.3 Common causes of periureteral masses and their imaging findings
Retroperitoneal Fibrosis
Retroperitoneal fibrosis is a rare entity with an estimated incidence of 1 in 200,000, most often found in middle-aged men [16]. The typical age range is 40–60, while men are affected two to three times more frequently than women [16]. Two thirds of cases of retroperitoneal fibrosis are considered primary or idiopathic, so-named Ormond’s disease [17]. Remaining cases are secondary to primary insults such as external beam radiation, trauma or infection, and certain drugs including methysergide, ergotamines, hydralazine, and phenacetine.
Retroperitoneal fibrosis tends to present early as a mantle of soft tissue surrounding the distal aorta near the level of L4 and L5, often enveloping the aortic bifurcation and common iliac arteries. As tissue extends laterally, the ureters—often bilaterally—become encased and classically deviated medially by the retractile effect of the fibrotic tissue. Depending on stage, soft tissue may extend far superiorly along the retroperitoneal axis to involve the duodenum, pancreas, and upper abdominal arteries (SMA, renal arteries, etc.). With more extensive disease, the ureters may become obstructed. Distinguishing benign retroperitoneal fibrosis from malignancy such as lymphoma is difficult and occasionally impossible. Nevertheless, benign retroperitoneal fibrosis tends to encase the aorta, IVC, and ureters, while lymphoma tends to encase and also elevate the aorta and IVC off the spine.
On CT, fibrotic soft tissue generally is isodense to muscle and demonstrates enhancement based on the disease activity: brisk enhancement may be seen with active inflammation while weak or no enhancement may be detectable in the chronic phase. Excretory phase imaging is appropriate in the setting of hydronephrosis, so that potential sites of ureteral obstruction can be localized.
On MR, fibrotic tissue tends to demonstrate low signal on T1- and T2-weighted imaging, except in the acute inflammatory phase when high signal on T2-weighted images may be seen reflecting edema. Dynamic postcontrast imaging with gadolinium generally mirrors T2 signal changes: early enhancement in the presence of active inflammation but weak delayed enhancement in the chronic indolent phase.
Lymphoma and Metastases
High on the “short list” of differential diagnoses for retroperitoneal masses are lymphoma and metastases. Urinary tract involvement by lymphoma and metastases are usually accompanied by generalized retroperitoneal disease, while isolated periureteral lymphoma and metastases are quite rare. Potential primary causes of periureteral metastases include primary neoplasms of the prostate, gastrointestinal tract, breast, and lung, as well as melanoma [18].
Lymphomatous involvement of the renal pelvis and ureter are usually found in the setting of direct lateral extension from adjacent retroperitoneal disease, less commonly from direct contiguous spread from renal and perirenal lymphoma (Fig. 21.15) [18]. Isolated peripelvic and periureteric lymphoma are very rare. Confident diagnosis of retroperitoneal lymphoma is rarely difficult, given the vast majority present as bulky, lobulated, sometimes confluent nodal masses. In cases where retroperitoneal fibrosis and lymphoma are both considered, helpful clues to a diagnosis of lymphoma include the following: any non-retroperitoneal site of nodal disease (mesentery, spleen, chest, pelvis, etc.), ureteral displacement rather than retraction/stenosis, and elevation of the aorta and IVC off the spine.
It is important to note that not all bulky retroperitoneal lymphadenopathy equals lymphoma, and metastatic disease must also be considered. Consideration of a primary malignancy is largely directed by known history and epidemiologic risk factors. In young adult men with periureteral or perirenal lymphadenopathy, a primary testicular malignancy (seminoma in particular) must be excluded. In middle-aged and older women, ovarian metastases may produce similar lymphadenopathy. In both cases, lymphatic spread along the gonadal vein may produce marked periureteral lymphadenopathy due to the close proximity between the gonadal vein and ureter.
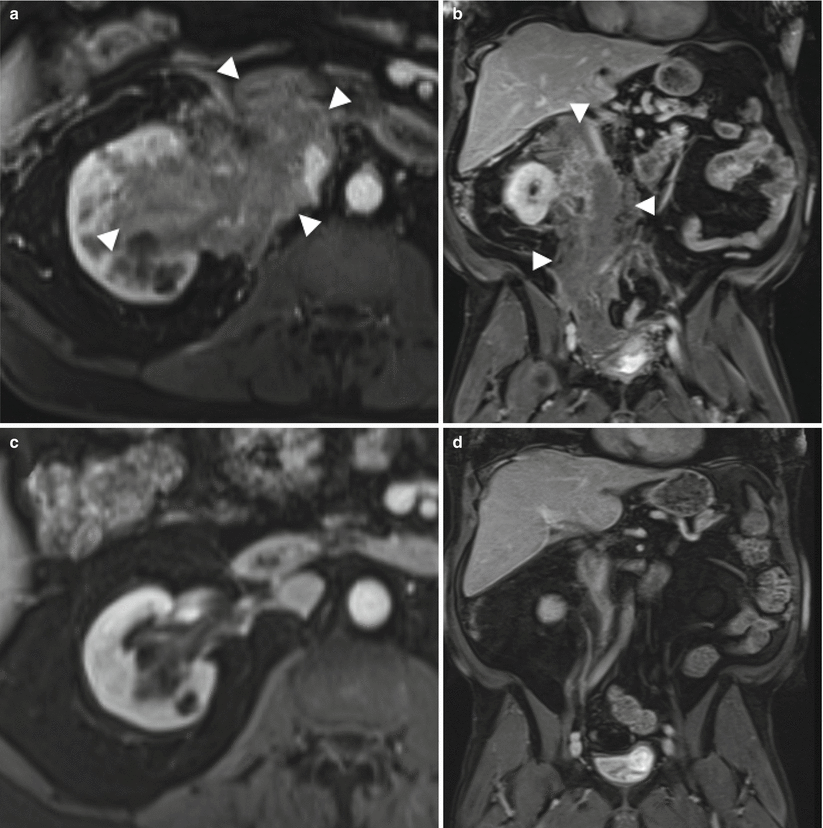

Fig. 21.15




Periureteral masses: Lymphoma/myeloma. Axial and coronal T1+Gd MR. (a, b) August 2011. Extensive enhancing soft tissue mass encasing entire length of the right ureter and renal pelvis, infiltrating the kidney. Histology revealed plasma cell dyscrasia consistent with multiple myeloma (demarcated by arrowheads). (c, d) October 2011. Dramatic improvement following chemotherapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree