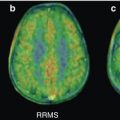
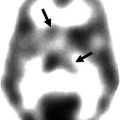
Fig. 43.1
Examples of the ability of MET-PET to differentiate between tumor progression/recurrent tumor and radionecrosis. Left images, MRI; middle images, MET-PET; right images, fusion. (a) Tumor progression and (b) radionecrosis
Table 43.1
Studies evaluating the role of MET-PET in the follow-up of gliomas and in the differentiation between recurrent tumors and pseudoprogression/radiation necrosis
Author [ref] | Year | N | Patients | Results/remarks |
|---|---|---|---|---|
Li et al. | 36 | Suspected residual/recurrent tumor; HPEa and follow-up: recurrence in 28 | Sensitivity 96 %, specificity 88 %, accuracy 94 % | |
Tripathi et al. | 37 | Evaluation of recurrent diseases; final diagnoses: recurrence (24), no recurrence/stable disease (11) | Sensitivity 95 %, specificity 89 % (using a cutoff T/N ratioa >1.9; good interobserver agreement; straightforward tumor localization | |
Grosu et al. | 42 | Pretreated gliomas (29) or brain metastases (13) | Sensitivity 91 %, specificity 100 %; useful for differentiation of residual/recurrent tumor from treatment-related changes/pseudoprogression | |
Okamoto et al. | 29 | Suspected of recurrent brain tumors by MRI after radiation; HPE or follow-up: recurrence (22), radiation necrosis (11) | T/N ratio for recurrence 1.98, for necrosis 1.27; high diagnostic value even for partial volume effect-affected small lesions; early diagnosis of recurrent tumor possible | |
Dandois et al. | 28 | High-grade glioma; comparison with perfusion MRI | Equal performance of rCBVa and MET-PET for differentiation necrosis from recurrence tumor | |
Kim et al. | 10 | High-grade gliomas after therapy; final diagnoses by clinical follow-up or HPE: radiation necrosis (6), recurrence (4). Comparison with MRI and FDG | rCBV higher in the recurrence group; no difference between groups in T/N ratio; perfusion MRI superior to MET-PET | |
Yamane et al. | 69 | Initial histopathologic diagnoses: primary tumor (39), metastatic tumor (30). Final diagnosis by HPE, clinical and radiological follow-up | Sensitivity 83 %, specificity 82 %, accuracy 88 %; change in intended management by the scan results in 45 % | |
Terakawa et al. | 77 | Glioma (26) and metastatic tumors (51) treated with radiotherapy after primary treatment; final diagnoses in lesions: recurrence (40), necrosis (48) | T/N mean ratio most informative index for differentiation. T/N mean ratio >1.41: sensitivity79 %, specificity 75 % for metastases. T/N mean ratio >1.58: sensitivity and specificity 75 % for gliomas | |
Pötzi et al. | 28 | Glioblastoma multiforme after surgical and/or conservative treatment | Focally increased uptake in 24 patients; no prognostic information; not superior to conventional imaging | |
Grosu et al. | 39 | Resected malignant gliomas; comparison with MRI | Size and location of residual MET uptake differ from abnormalities found on MRI; MET-PET can help in differentiation and in outlining the gross tumor volume with greater accuracy | |
Van Laere et al. | 30 | Treated gliomas: ACa grades II–IV (23), ODa (4), mixed oligo-astrocytomas (3); suspected recurrence/progression | Increased uptake in 28; interobserver agreement 100 %; best prognostic predictor in the subgroup of AC | |
Tsuyuguchi et al. | 11 | Suspected glioma recurrence; final diagnoses: recurrence (6), radiation necrosis (5) | Sensitivity in detecting tumor recurrence 100 %, specificity 60 %, accuracy 82 %; no significant differences in T/N ratio or SUV | |
Tsuyuguchi et al. | 21 | Suspected metastatic tumor recurrence; final diagnoses: recurrence (9), radiation necrosis (12) | Sensitivity in detecting tumor recurrence 78 %, specificity 100 %; significant differences in T/N ratio and SUV | |
Sonoda et al. | 10 | Suspected recurrence on MRI; clinical diagnoses: recurrence (5), radiation necrosis (7) | All recurrent tumors showed MET uptake; false-positive MET uptake in 1∕7 radiation necrosis cases | |
Viader et al. | 1 | Clinically suspected recurrence OD | High uptake of MET; CT and MRI normal | |
Ogawa et al. | 15 | Suspected recurrence, final diagnoses HPE (14): necrosis (5), recurrence (10) | No false positives on MET; all recurrences detected by MET | |
Lilja et al. | 4 | Long-term high-grade glioma survivors (2 with recurrence) | Tumor extent in both patients with recurrence larger than on CT; false-positive uptake in 1 clinically stable patient |
The uptake of methionine in both conditions is different. As in primary gliomas, the uptake in recurrent tumor lesions is caused by active uptake of methionine in the tumor cells. On the other hand, radiation injury leads to passive diffusion across a broken blood-brain barrier. Theoretically, the uptake in tumor cells should exceed the uptake after radiation injury; however there is a possibility of overlap between this uptake.
In general, most studies agreed that MET-PET is useful in correctly identifying tumor recurrence after radiation. Li et al. recently published their findings of MET-PET in 36 postoperative patients with suspected residual/recurrent tumors, of which, after histopathologic examination and/or clinical follow-up for more than 6 months, 28 were verified having recurrent gliomas. The sensitivity, specificity, and accuracy of MET-PET were 96.4 %, 87.5 %, and 94.4 %, respectively (Li et al. 2012). Tripathi et al. scanned 24 patients with tumor recurrence and 11 patients without recurrence with MET. Using a cutoff T/N ratio of >1.9 to differentiate recurrence from no recurrence, sensitivity was 94.7 % and specificity 88.9 %. The interobserver agreement was good, with a kappa-value of 0.93 (Tripathi et al. 2012). Another study, from Grosu et al., reported a sensitivity of 91 % and a specificity of 100 % for differentiating tumor tissue and treatment-related changes in 29 patients with pretreated gliomas and 13 patients with pretreated brain metastases (Grosu et al. 2011). Okamoto et al. scanned 29 patients with 33 lesions suspected of recurrent brain tumors by MRI after radiation therapy. They found a significant higher T/N ratio in recurrence (1.98 ± 0.62) compared to necrosis (1.27 ± 0.28) and a high diagnostic value even for partial volume effect-affected small lesions (Okamoto et al. 2011). Terakawa et al. scanned in total 77 patients with MET, 51 patients who had been previously treated with radiotherapy after primary treatment for metastatic brain tumor and 26 after treatment for glioma. Best sensitivity and specificity results were found with a T/N ratio (by SUVmean) of 1.58 for gliomas (sensitivity and specificity 75 %). For metastatic brain tumors, L/N greater than 1.41 provided best sensitivity and specificity (79 % and 75 %, respectively) (Terakawa et al. 2008). Yamane et al. asked the referring clinicians their treatment strategy before and after the MET-PET. A change in intended management by the scan results was found in 45 % of the patients (Yamane et al. 2010). Van Laere et al. scanned 30 patients with treated gliomas of whom 28 were positive, leading to a high sensitivity. Moreover, the interobserver agreement was 100 %, and MET-PET was the best prognostic predictor in a subgroup of astrocytomas (23 patients) (Van Laere et al. 2005). Earlier studies showed MET uptake in all recurrent tumors and one false-positive scan in seven patients with radionecrosis (Sonoda et al. 1998), and a case report mentioned high uptake of MET in a recurrent tumor with CT and MRI being normal (Viader et al. 1993). A study by Lilja et al., already performed in 1989, showed a larger tumor area than CT in two patients with residual tumor; however they also found nonspecific uptake in one clinically stable patient assumed to have no recurrence (Lilja et al. 1989).
Three other studies also published good sensitivity and specificity results; however they claimed that MET-PET was not better than conventional imaging. Pötzi et al. did find focally increased uptake in 24 out of 28 patients with treated glioblastoma multiforme, but showed no added prognostic information of the MET-PET, therefore in no way being superior than conventional imaging (Potzi et al. 2007). Kim et al. stated that perfusion MRI is superior in distinguishing a recurrence of high-grade glioma from radiation necrosis than MET-PET (Kim et al. 2010). Dandois found that perfusion MRI had an at least equal performance to MET-PET in differentiating tumor recurrence and necrosis in high-grade gliomas by using the values determined from regional cerebral blood flow (Dandois et al. 2010).
43.6 Conclusions
A summary of the role of 11C-methionine in the imaging of gliomas can be found in Table 43.2. In general, MET-PET is useful in diagnosing brain tumors with good sensitivities and specificities, has prognostic value, plays a role in defining tumor extent, and may help in biopsy and radiotherapy planning. For the use of MET-PET in grading primary brain tumors, there is not enough evidence at the moment yet.
Table 43.2
A summary of the role of 11C-methionine in the imaging of gliomas
Gliomas | Positive findings | Negative findings |
|---|---|---|
Diagnosis | High sensitivity and specificity | False positives and false negatives may occur |
High positive predictive value | Different cutoff ratios used | |
Low negative predictive value | ||
If MET-PET is positive → high probability of brain tumor | ||
T/N ratio improves results | ||
Grading | Useful to differentiate between low- and high-grade gliomas | Not accurate enough to divide gliomas in different grading scales |
Prognosis | Important prognostic tool | |
May be used as survival factor | ||
Lower MET uptake → better prognosis | ||
Defining tumor extent | Valuable role, better than CT and MRI | |
Biopsy planning | Added value for best localization demonstrated | |
Radiotherapy planning | Alteration in target volume demonstrated | |
Adequately delineates tumor volume | ||
Differentiation between recurrence and radionecrosis | High sensitivity and specificity | Overlap in uptake values in both situations |
High diagnostic accuracy | ||
More useful than CT and MRI |
In the differentiation between tumor recurrence and radiation necrosis, most studies have in common high sensitivity, specificity, and diagnostic accuracy for MET-PET, with advantages over conventional imaging. However, there still could be overlap in the uptake values of methionine in these two entities.
One of the major limitations of MET-PET is the use in studies of different cutoff values in T/N ratios and used reference regions for the calculation of this ratio. The precise location of this reference region is important as local variations in methionine uptake may significantly alter the result. Therefore, a normal methionine uptake map and clear location of reference region would be very useful to improve sensitivity. A start for this normal database is already performed by Coope et al. (2007) but should be extended and available worldwide.
Of course, the recent development of hybrid PET/MRI cameras could lead to a jump forward in the imaging of brain tumors and metastases. Especially simultaneous PET/MRI systems have general advantages, such as recording of dynamic phenomena, absolute match between tissue information of both modalities under the same physiological conditions, use of contrast-enhanced MRI information in the pharmacokinetic modeling of the PET data, better localization of the PET signal within the soft tissues, and one-stop-shop principle for the patients (Glaudemans et al. 2012). Especially in brain imaging, the use of contrast-enhanced MRI together with PET could solve the aforementioned problems. Recently, the first two studies in this field were published, not with the simultaneous PET/MRI camera but both with an inserted PET system within a conventional 3T MRI scanner. The first study showed that structural, functional, and molecular imaging in patients with brain tumors was feasible with diagnostic imaging quality (Boss et al. 2010). The second study used a brain PET insert/MRI system in 50 patients with intracranial masses, head and upper neck tumors, or neurodegenerative diseases. The MRI image quality was uncompromised and a high accordance of PET results was found between PET/MRI and PET/CT. We are curiously waiting for the first study that uses simultaneous PET/MRI imaging in brain tumors and metastases.
Acknowledgment
The authors want to thank Dr. Annemiek M. R. Walenkamp for her help in writing this book chapter.
References
Barnholtz-Sloan JS, Sloan AE, Davis FG et al (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree