and Ashutosh Dash2
(1)
Nuclear Security and Isotope Division, Oak Ridge National Laboratory, OAK RIDGE, USA
(2)
Isotope Production and Applications Division, Bhabha Atomic Research Centre, Mumbai, India
12.1 Introduction
Bone pain from skeletal metastases resulting from many types of primary tumors is a common debilitating issue which greatly decreases the quality of life (Paes et al. 2011; D’angelo et al. 2012; Finlay et al. 2005; Gough et al. 2014; Jansen et al. 2010; Knapp and Baum 2012; Mantyh 2014; Ogawa et al. 2012; Paes et al. 2011). Such metastases result from tumor cells entering the circulation and lodging in the richly oxygenated bone marrow with subsequent proliferation into metastatic sites. Such bone pain resulting from skeletal metastases of primary tumors is often encountered in patients presenting with primary tumors of the prostate, breast, small cell lung, and other cancers (D’angelo et al. 2012; Gough et al. 2014; Lewington 1993; Mantyh 2014; Ogawa 2012; Jansen et al. 2010). The hallmarks are destruction of bone tissue, marrow crowding, fractures, and distinct localized chemical changes. Although not well understood, the sensation of bone pain generally results from stimulation of specialized nociceptor pain-sensitive nerve fibers that innervate bone tissue. These pain signals are relayed from the sensory neurons to the brain, creating the sensation of pain, which originates from both the periosteal pressure and from the bone marrow. Activation of nociceptors responsible for bone pain results from a variety of mechanisms which include the deterioration of surrounding tissue and bone destruction. This is a debilitating situation which can dramatically affect mobility and quality of life.
Patients with these skeletal metastases very often present with bone pain for which the traditional first-line treatment has been the use of opiates. Often teletherapy and also chemotherapy with bisphosphonates are also used, often in combination. These methods are often only partially effective and have their own drawbacks. Although whole-body or hemibody irradiation has been used, because of the effects of gastrointestinal damage, these approaches are not often used, especially for multiple metastases juxtaposed to the viscera. Localized irradiation is effective for localized sites such as limbs, where radiation damage to normal tissue is minimized and dose levels of 8 Gy are used with a prescription of up to 40 Gy for multiple sessions. The use of bisphosphonates is very common as one treatment option used alone or in combination for bone pain. While narcotics reduce the associated pain indirectly, the phosphonates act as a type of chemotherapy by inhibition and destruction of osteoclasts. Since these substances are not radioactive, no license or specialized facilities are required for their application.
12.2 Treatment of Metastatic Bone Pain with Therapeutic Radioisotopes
Since these strategies can have dramatic clinically relevant side effects, the use of therapeutic radioisotopes which localize at the metastatic sites and result in pain reduction is an attractive and effective alternative strategy generally with minimal side effects (Atkins et al. 1995; Bauman et al. 2005; Brady et al. 2013; D’angelo et al. 2012; Lewington 1993, 2005; Liepe et al. 2000a, b; Mantyh 2014; Morris and Scher 2003; Ogawa and Washiyama 2012; Paes and Serafini 2010; Paes et al. 2011; Robinson 1986; Silberstein 1993; Srivastava 2002). The first assessment of the use of a radioisotope for bone pain palliation was conducted by Charles Pecher, a Belgian physician working at the Lawrence Berkeley Laboratory as early as 1936, who produced 89Sr in the cyclotron and then conducted many experiments using this tracer to assess calcium pharmacokinetics and metabolism. He first reported the clinical use of 89Sr, which was the first report for use of a therapeutic radioisotope for the palliation of bone pain (Pecher 1942). Following his untimely suicidal death, this seminal scientific achievement was apparently forgotten by the medical community during the 1942–1993 period and not resurrected until the development and availability of Medastron®. However, sadly, no mention of his contributions for the use of 89Sr for bone pain palliation has been included in the commercial product literature. Radiopharmaceuticals used for the palliation of painful bone metastases should exhibit several key criteria including high tumor-to-normal bone ratio, in vivo stability, low bone marrow toxicity, rapid clearance from normal bone and soft tissues, ability to predict biodistribution patterns based on bone scintigraphy, and ease of preparation.
Most radioactive phosphonates chemisorb to the bone while the rhenium-labeled agents also appear to form an oxide species [i.e., oxidation of Re(V) to Re(VII] and chelate release) which attaches to the bone surface. Although the exact mechanism of action is not fully understood, the radiation released is felt to evidently sterilize those cells which release growth substances such as cytokines which results in periosteal pressure and nerve stimulation which causes the pain. It is curious that some authors refer to killing of skeletal metastatic tumor cells as the mechanism of action of the use of radioisotopes for bone pain palliation, although tumor reduction is generally not observed. The mechanism of action results from sterilization of cells which release inflammatory substances which activate the nociceptors. Both osteoblastic (new bone formation) and osteolytic (osteoclast breakdown, resorption/bone destruction) lesions are present in skeletal metastases. It should be noted that the goal of such treatments is to palliate the pain to manageable levels and thus to dramatically improve the quality of life. Although these methods in general do not eradicate the tumor metastases, there are promising recent results that therapeutic effects are possible with multiple repeat dosing (vide infra). The key radioisotopes which have been used or which are being evaluated for bone pain palliation are summarized in Table 12.1.
Table 12.1
Properties of radioisotopes and agents for bone pain palliation
Radioisotope agent | Production modea | Half-life | Particle emissions, Emax | ~Soft tissue penetration, mmmax | Principle gammas |
|---|---|---|---|---|---|
Commercially available bone pain palliation agents | |||||
Samarium-153 “Quadramet®” | R | 1.9 days | β, 0.81 MeV | 4 mm | 103 (28 %) |
Strontium-89 “Metastron®” | R | 50.5 days | β, 1.46 MeV | 7 mm | 910 (0.01 %) |
Rhenium-186b (Sn)HEDP “Etidronate” | R/A | 3.8 days | β, 1.07 MeV | 5 | 137 (9 %) |
Bone pain palliation agents reported in clinical trials | |||||
Phosphorus-32 Orthophosphate | R/A | 14.3 days | β, 1.71 MeV | 8.5 mm | None |
Yttrium-90 Citrate and bisphosphonate | R/F | 2.7 days | Β 2.27 MeV | 12 mm | None |
Iodine-131 “BPB3” | R | ||||
Rhenium-188 (Sn)HEDP | R | 0.7 daysc | β, 2.12 MeV | 10 mm | 155 (15 %) |
Lutetium-177 BPMAD/EDTMP DOTMP | R | 6.7 days | β, 0.497 MeV | ||
Strontium-85 | R | 64 days | CE, 10–15 keV | 10 mm | 514 keV |
Radium-223 Chloride “Xofigo®” | Decay | 11.4 days | CE – α’s 5.606 MeV 5.715 MeV | ||
Tin-117m DTPA | R, A | 13.6 days | CE, 126, 129, 151 keV | 158 keV | |
Thorium-227d EDTMP | 18.7 days | 18.7 days | α’s 5.757, 5.977, 6.038 MeV | ||
Lead-212e DOTMP | 10.2 h | 10.2 days | CE 334 keV | 234 keV | |
Because of the importance of evaluating the use of alternative more effective methods with minimal side effects, the use of beta-emitting radionuclides which target these sites of increased bone metabolism was evaluated as early as the late 1940s with 89Sr (Pecher 1942). Since strontium is categorized in Group IIA of the periodic chart and is a congener of calcium, the use of 89Sr was a good choice and can be reactor-produced by the neutron irradiation of enriched 88Sr (Davis et al. 2000). The use of 89Sr slowly gained interest and was approved by the US Food and Drug Administration (FDA) in 1993 based on the work of Robinson and others (Lewington et al. 1991; Quilty et al. 1994; Robinson 1986; Robinson et al. 1987). Because of the special role which beta-emitting radioisotopes were expected to play for the treatment of bone pain palliation, in the intervening time period a number of other candidates—most notably 32P (Silberstein 1993).
The phosphonates labeled with such radioisotopes concentrate in the bone as a function of the osteoblastic activity. Several 186Re-labeled agents have been evaluated for both bone pain palliation (De Klerk et al. 1996; Maxon et al. 1991) and osteosarcoma therapy (Bruland et al. 1996), and 153Sm agents (Singh et al. 1989; Turner et al. 1989) have also been developed. The radioisotopes are attached to the phosphonate-targeting agents which have been commercialized. More recently, several other beta-emitting radioisotopes—in particular 188Re and 177Lu—have entered clinical trials and have also shown promise for this type of application. Those agents which have been approved by the FDA (153Sm-EDTMP and 89Sr-chloride) provide a reported 40–95 % pain relief response beginning 1–4 weeks following initiation of therapy. Response can last up to 18 months, and in many patients the use of opiates and other analgesics is drastically reduced. Reversible marrow suppression is often observed as thrombocytopenia and neutropenia.
In addition, the use of radioisotopes which have very short range penetration such as 117mSn (conversion electron) and 223Ra (alpha emission) are also being explored for this application. However, in spite of the benefits of the use of these effective radioisotopic methods, first-line clinical treatment for bone pain continues to focus on traditional methods administered by oncologists, urologists, and radiologists, who have been trained in the use of these methods and who are often unaware of the benefits for referral for nuclear medicine therapy. In addition to benefits for palliation, the use of radioisotopes has also been shown in some studies to provide therapeutic effects.
The goal of this chapter is to describe the development and use of a variety skeletal metastatic site-specific radiopharmaceuticals for the palliative treatment of bone pain. One important point is that many of these radioisotopes for bone pain palliation are neutron-rich and CE-/beta-particle-emitting radioisotopes available from reactor production, as described in Chap. 2. This illustrates the further importance of research reactors for the availability of many radioisotopes which are used in both unsealed radiopharmaceutical source (nuclear medicine) and sealed source (radiation oncology/interventional radiology–cardiology) applications. Agents which are commercially available are briefly discussed since these radiopharmaceuticals are well documented in the literature. Agents which are currently under development and those in clinical trials are discussed in more detail.
Important practical issues for radioisotope choice include availability and costs and also the half-life and thus the dose rate for use in outpatient therapy, depth of particle penetration, and emission of gamma photons for imaging. Availability and costs revolve around the required production and processing (See Chaps. 5, 6, 7, and 8) and other practical and economic factors. The radioactive agents used for bone pain palliation generally consist of either simple radioactive ionic species such as 32P (as the phosphate anion) and 89Sr and 223Ra, as the divalent M+2 cations. These species target the bone because of their periodic similarity to natural constituents. Other radioactive agents or carrier molecules used for bone targeting include phosphonates (−C-P-C-) to which the radioisotopes are chemically attached and include 153Sm EDTMP, 186/188Re HEDP, and 177Lu-EDTMP.
12.3 Commercially Available Beta-Particle-Emitting Approved Agents for Bone Pain Palliation
12.3.1 Rhenium-186 HEDP
Substituted phosphonate (Fig. 12.1) complexes represent attractive carrier molecules for targeting therapeutic radioisotopes to skeletal regions of increased osteolytic metabolism. Rhenium is a Group VIIb transition metal, and the 186Re hydroxyethylidene diphosphonate (HEDP) complex, as an example, was developed in the 1980s for bone pain palliation and, in contrast to the traditional use of 89Sr, had the added advantage of gamma photon emission (Table 12.1) which permits evaluation of localization with gamma camera imaging. It was expected that clinical use of this agent would rapidly progress but only became available locally on a physician prescription basis in Europe and was never approved for broad routine use because of apparent regulatory issue.
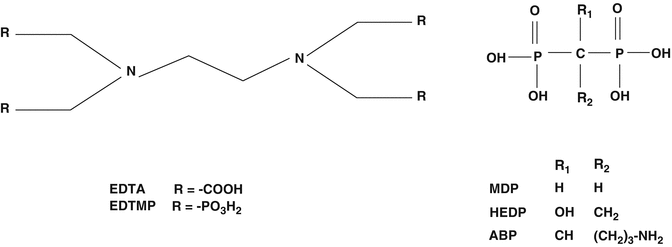

Fig. 12.1
Phosphonate carrier ligands for bone pain palliation agents
Although preparation of 186Re-HEDP had been well established, the relatively low specific activity of reactor-produced 186Re was a key requirement for preparation of the HEDP complex which was not initially realized. This was later established where only soft tissue uptake rather than skeletal localization was detected during evaluation of 188Re HEDP prepared using very high specific activity no-carrier-added generator-produced 188Re (Dash and Knapp 2015). In contrast, when carrier rhenium is added to similar levels encountered during the use of low specific activity 186Re, the expected targeted skeletal localization is realized (Lam et al. 2008). In studies reported by Edler et al., the apparent reason for these observations is the unknown chemical structure of the Re(V)-HEDP species, which apparently consists of Re-Re bonds and is formulated as an oligomer (Elder et al. 1997). The authors used extended X-ray absorption fins structure (EXAFS) analysis to evaluate the species formed using nonradioactive rhenium and have formulated that the species formed in the presence of high levels of the stannous ion reductant probably represents a tetramer of rhenium atoms which are bridged by the HEDP ligands. This structure evidently also binds an equivalent number of tin atoms as well as additional HEDP ligands. Based on the levels of the stannous ion-reducing agent used, different species are apparently formed. When an excess of the stannous ion-reducing agent is used, the species formed appears to have the composition Li(x)Re4(OH)2Sn4HEDP12. Thus, 186Re-HEDP and 188Re HEDP described below are examples of agents which show specific targeted uptake but which have unknown chemical structures.
12.3.2 Samarium-153 EDTMP (“Quadramet®”)
Samarium-153 is reactor-produced by the neutron irradiation of enriched 152Sm targets (Sartor 2004) and is incorporated into the phosphonate entity, referred to as lexidronam (Fig. 12.2). With the emission of a moderate energy beta particle and an excellent half-life for outpatient applications, 153Sm was evaluated as an alternative to 89Sr for bone pain palliation (Bauman et al. 2005; Finlay et al. 2005; Silberstein 2005). Another advantage—which probably has not been fully appreciated until in more recent times—is the emission of a gamma photon, which permits the use of 153Sm to monitor uptake/targeting, biokinetics, and evaluation of dose delivery in relation to therapeutic response. In the current developing era of “personalized medicine,” the use of dual beta-/gamma-emitting radioisotopes for these and other therapeutic applications thus offers several advantages.
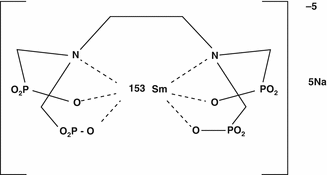

Fig. 12.2
Chemical structure of lexidronam
12.3.3 Stronium-89 Chloride
Since both strontium and calcium are members of Group IIA the use of 89Sr as a surrogate of calcium was an early strategy which had been explored and 89Sr is still used for bone pain palliation. The evaluation of 89Sr as a targeted agent for bone pain palliation was initially reported in Germany in 1946. This agent was later approved in the US by the Food and Drug Administration (FDA) as “Metastron®” in 1993 and has since that time been a widely used agent for bone pain palliation.
12.4 Examples of Bone Pain Palliation Agents under Development and in Clinical Trials
In addition to the widespread use of the commercial regulatory-approved agents described above, in particular 153Sm and 89Sr, there are also several other palliative agents radiolabeled with beta-emitting radioisotopes described below which have shown promise for bone pain palliation and are being used in patient trials.
12.4.1 Iodine-131
Another diphosphonate option for bone pain palliation utilized 131I, which is a readily available and well-established therapeutic radioisotope. Preparation and studies in a rat model were initially reported with the 131I-labeled α-amino-(4-hydroxybenzylidene)-diphosphonate (BPB3) agent (Fig. 12.3) (Eisenhut 1984). Iodine-131 radiolabeling was performed in about 95 % yield with a BPB3 specific activity of about 50 mCi/mg by electrophilic aromatic substitution using the iodate anion in 1 N HCl. The biokinetics, dosimetry data, and pain response were reported in initial studies in 18 patients with skeletal metastases from prostate, breast, and other primary carcinomas receiving doses from 6–48 mCi of 131I-BPB3 (Eisenhut et al. 1986a). In further studies, these authors then pursued evaluation of several structurally related radioiodinated benzylidenediphosphonates (BDP) with alpha and para position substitutions including -H, −OH, and -NH2 (Eisenhut et al. 1987). The 4-methoxy- and 4-nitro-substituted diphosphonates were prepared by phosphorous acid treatment of the respective benzonitriles in the presence of phosphorus bromide (PBr3). The 4-hydroxy and 4-nitro derivatives were then prepared by hydrolytic HBr cleavage and subsequent catalytic hydrogenation. Transformation of the 2-amino to the 2-hydroxy derivative was accomplished by treatment with sodium nitrate in HCl. The proposed structures of these derivatives were confirmed by chromatography, spectroscopy, and elemental analysis and were then radiolabeled with 131I. Further studies of these BDP analogs were focused on the comparative biokinetics and bone uptake values in Sprague Dawley rats. Total-body retention measurements of α-amino-(3-[131I]iodo-4-hydroxybenzylidene)diphosphonate analog revealed an effective half-life of the activity of 169 h. LD50 measurements in rats amounted to 64 mg/kg. In addition, autoradiographic analyses were performed in bone samples obtained from osteosarcomic SD rats following intravenous administration (Eisenhut et al. 1986b). Apparently, these agents have not been evaluated further and subsequent patient trials have not been reported.
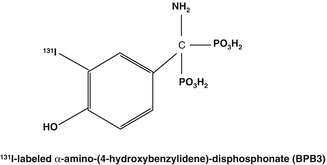

Fig. 12.3
Iodine-131-labeled benzylidene diphosphonate structures
12.4.2 Phosphorus-32
The bone is constructed of a complex hydroxyapatite matrix in which phosphorus plays an integral role. The use of beta-emitting 32P as the phosphate had thus been an obvious candidate to evaluate for bone pain palliation since it mimicked the natural cation and has been cost-effectively available on a broad scale. In an IAEA-coordinated multicenter study (Fettich et al. 2003) to evaluate safety and efficacy of 32P for palliation of bone pain due to bony metastases by comparing it to 89Sr, or bone pain palliation, 93 cancer patients with osteoblastic bony metastases were included into the study of which 48 were treated by 89Sr (150 MBq) and 45 by 32P (450 MBq). The results of this study suggested that 32P is slightly but not significantly less effective than 89Sr for palliation of bone pain due to bony metastases. Although 32P appears to be more toxic it is important to note that no toxic effects requiring specific treatment were seen in either group. This study inferred that 32P was as safe as 89Sr using doses up to 450 MBq (Fettich et al. 2001). The results of this study are expected to shed further light on the perspectives of 32P for patients with painful skeletal metastases. In light of the cost-effective availability, widespread use of 32P should be encouraged to reduce cost of medical care of patients with intractable bone pain due to cancer metastases, but further widespread use of 32P for bone pain palliation has not been reported.
12.4.3 Yttrium-90-Labeled Citrate and EDTMP
Very early during the assessment of beta-emitting radioisotopes for bone pain palliation 90Y had been evaluated as a high-energy beta particle-emitting radioisotope for this application as the citrate complex but had not been subsequently widely used. Renewed interest in the use of 90Y, however, resulted in the commercial release of the Multibone® agent (EDTMP) in central Europe for radiolabeling with 153Sm, 90Y, 111In, and188Re (Mitterhauser et al. 2004a, b). In this case, the strategy was to make available a common kit which could be used for radiolabeling with a variety of radionuclides. In the case of bone pain palliation, the use of beta emitters with different beta energies would permit the tailoring of irradiation of diagnosed metastases to minimize damage to normal tissue (Bouchet et al. 2000; Lewington 2005). The commercially available multibone EDTMP kit agent consists of EDTMP, SnCl2, ascorbic acid, and glucose (Mitterhauser et al. 2004a, b). The 90Y-DOTA conjugated 4-amino-1-hydroxybutylidene-1,1-bisphosphonate (90Y-DOTA-HBP) (Fig. 12.4) has also been prepared and biodistribution studies compared with 90Y-citrate in mice (Ogawa et al. 2009). The HBP analogs demonstrated much faster blood and most soft tissue clearance and higher bone accumulation. The advantage of further studies and potential human use of the HBP analogs is the very stable Y+3-DOTA complex, which would not release free 90Y+3 for targeting to normal bone.
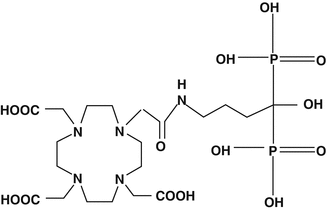

Fig. 12.4
DOTA conjugated 4-amino-1-hydroxybutylidene-1,1-bisphosphonate (DOTA-HBP)
12.5 New Radiolabeled Agents Being Developed for Bone Pain Palliation
In addition to the use of 186Re, 153Sm, and 90Sr as the three major commercially available bone pain palliation agents, there are a variety of other beta-emitting radioisotopes which are being evaluated for bone pain palliation. The results from several clinical trials have been reported with several of these agents suggest their potential utility for further evaluation and potential routine clinical use.
12.5.1 Rhenium-188
Because of its availability from the 188W/188Re generator which has a useful shelf-life of several months (Dash and Knapp 2015), the use of 188Re-HEDP offers many benefits. In addition to similarity to the established use of 186Re-HEDP, the 188Re-HEDP has been extensively evaluated on a clinical trial basis with extensive discussion in the literature. Within the basic science and clinical research communities, 188Re-labeled bone pain palliation agents have by far seen the greatest progress.
12.5.1.1 Rhenium-188 (V) DMSA
Since both Tc and Re are transition metal members of Group VIIB of the periodic chart and have similar oxidation states and chemistry, many 188Re-labeled radiopharmaceuticals are based on their 99mTc analogs. Meso-2,3-dimercaptosuccinic acid (DMSA) represents excellent ligand properties for formation of many different metal ions, including Tc and Re (Stanik et al. 2012). A tri-anionic Tc(III)-DMSA complex is prepared by acidic reduction of pertechnetate which has been widely used for renal imaging. The 186Re-DMSA agent was prepared using the standard kit and stannous ion reduction at 100 ° C (Bisunadan et al. 1991), and this agent demonstrated localization for imaging of medullary carcinoma thyroid cancer and a variety of other tumors. In contrast, carefully controlled stannic ion reduction of pertechnetate at high pH (sodium bicarbonate) reacts with DMSA to form the quite different stable mono-anionic Tc(V)-DMSA complex which showed good targeting to bone metastases (Lam et al. 1997) which was a prelude for the evaluation of the 188Re congener for palliation.
These data led to the preparation and evaluation of the corresponding 188Re-DMSA for evaluation for the palliative treatment of skeletal lesions. In this case a basic pH of the reaction mixture is not required, and a range of pH values form the single mono-anionic Re(V)-DSMA species (Fig. 12.5) which is prepared by stannous ion reduction of perrhenate in the presence of the commercially available DMSA kit by heating to 100 ° C (Singh et al. 1993).
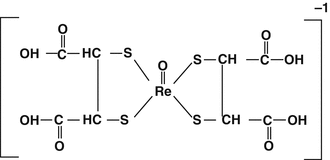

Fig. 12.5
Rhenium(V)-DMSA isomers
The presence of these isomers was confirmed by X-ray diffraction (Singh et al. 1991). Evaluation in animal models with subsequent patient studies demonstrated the excellent targeting of this agent in skeletal metastases from prostate cancer (Blower et al. 1998). Since DMSA exists in the meso (R, S) and racemic (DL) isomers, the mono-anionic product consisting of three species were separated by subsequent careful HPLC chromatographic analyses. Preparation of 186Re(V)DMSA requires a DMSA/SnCl2/Re ratio of 10:5:1 at 100 °C for 30 min (Singh et al. 1993). The chemistry of Re(V)-DMSA has been discussed in detail (Blower and Prakash 1999). Evaluation of the individual 188Re-labeled DMSA isomers showed only minor differences in skeletal uptake and thus established that isomer separation was not required for isolation of the most favorable isomer (Guhlke et al. 2009). Subsequent studies evaluated a new kit for preparation of the 188Re(V)-DMSA (Pirmettis et al. 2001).
Initial biological studies with the kit-based preparation of 188Re(V)-DMSA in animals and humans provided biodistribution, excretion, and dosimetry data on which further therapeutic studies could be based (Blower et al. 1998). Data from three patients who presented with skeletal metastases from primary prostate cancer showed and three with bronchus cancer with bone metises confirmed by standard skeletal imaging were imaged 3 and 24 h following administration of 370 MBq of the 188Re-(V)-DMSA. Clearance of 188Re was evaluated by blood and urine analyses. Normal bone uptake was similar to soft tissue and bone metastases demonstrated good targeting, with kidney uptake also observed. These preliminary findings then lead to subsequent detailed studies comparing the 99mTc and 188Re congeners for comparison of the tumor/normal tissue and kidney/soft tissue ratios in a group of 10 prostate cancer patients with skeletal metastases and to determine if imaging with 99mTc-DMSA could predict the targeting and subsequent therapy of lesions with 188Re-DMSA (Blower et al. 2000a, b, c). Imaging at 4 and 24 h after administration demonstrated a high linear correlation between the two agents. These data suggest that traditional tumor imaging could be used to estimate tumor and kidney radiation dose which would be delivered by the use of 188Re(V)-DMSA for bone pain palliation. Although this agent showed great promise for broader evaluation of patients presenting with skeletal metastases of other tumors, this agent is not evidently being evaluated further.
12.5.1.2 Rhenium-188 HEDP
Because of the early success and expected broad clinical use of 186Re-HEDP, 188Re HEDP was further developed and evaluated as an attractive analog (Lambert and de Klerk 2006; Liepe et al. 1998, 1999a, b, c, 2000a, b, c, 2001, 2002, 2003a, b, c, d, e, 2005a, b, 2009; Lin et al. 1997; Palmedo et al. 1997, 1998a, b, 1999a, b, 2000, 2002, 2003a, b; Maxon et al. 1998; Orsini et al. 2012; Savio et al. 2001; Scheffler et al. 2003, Zhang et al. 2003). The 188Re is available carrier-free from the 188W/188Re generator system (Hsieh et al. 1996; Pillai et al. 2012; Knapp 1998; Knapp et al. 1994, 1997, 1998, 2000, 2004; Jeong and Chung 2003; Dash and Knapp 2015). An extensive number of clinical trials with 188Re-HEDP have been described and demonstrate the excellent and cost-effective use of this agent for treatment of skeletal bone pain. In addition, several “kit” formulations have been developed for routine radiopharmacy preparation of this agent (Schmaljohan et al. 2000; Verdera et al. 1996, 1997a, b; Lin et al. 1997), and a detailed typical kit formulation strategy is depicted in Scheme 12.1.
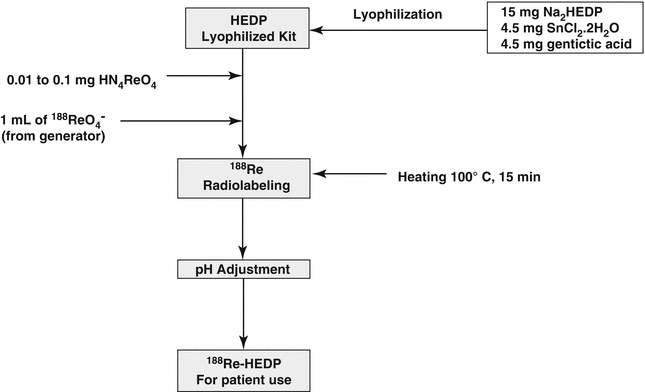

Scheme 12.1
Preparation scheme for 188Re-HEDP
As described earlier, preparation of 188Re-HEDP requires that the specific activity of the no-carrier-added 188Re-perrhenate obtained by NaCl elution of the 188W/188Re generator system be significantly reduced by addition of carrier perrhenate (ammonium perrhenate) prior to stannic ion reduction in order to form the requisite oligomeric product which shows localization in skeletal metastases (Verdera et al. 1997a, b; Inoue et al. 1981). Preparation of 188Re-HEDP using high specific activity 188Re-perrhenate forms a product which only shows soft tissue uptake (Bordoloi et al. 2015). Autoradiographic studies of skeletal samples from rats administered with 188Re-HEDP showed that the uptake of rhenium-188-HEDP in cortical bone was 33.5 % and in trabecular bones was 66.5 % after 4 h, 34.6 and 65.4 % after 24 h, and 35.9 and 64.1 % after 48 h, respectively. In bone metastases, an inhomogeneous distribution with a minimal and maximal tumor to non-tumor ratio of 3:1 and 14:1 after 4 h, 5:1 and 14:1 after 24 h, and 5:1 and 16:1 after 48 h was observed (Liepe et al. 2009), which probably has broad implications on the localization of other radiolabeled phosphonates and will provide some insight into the dosimetric considerations.
Rhenium-188-HEDP is generally prepared using 10 μmol of carrier Re, and drug composition may not only affect stability of the final drug product but may also influence bone affinity and subsequent efficacy. Since there are no standardized preparation methods, however, a study by ter Hein and co-workers reported the first GMP preparation of 188Re-HEDP production using stocks of sterile nonradioactive starting materials (ter Heine et al. 2014). On-site GMP production has the obvious advantage to allow rapid availability of this agent for treatment of patients who present with unmanageable pain. The production of GMP grade 188Re-HEDP will allow application of this therapeutic radiopharmaceutical in routine clinical practice and for support of clinical studies.
A more recent study by the same authors evaluated bone uptake in mice as a function of Re carrier concentration. Since the exact stoichiometric requirements for carrier Re had not been defined, the influence of Re carrier concentration during the preparation of 188Re-HEDP was investigated for the first time (Lange et al. 2015), by studying product radiochemical purity, in vitro affinity of the 188Re-HEDP for hydroxyapatite (HA) affinity, and in vivo bone accumulation in mice. Both HA binding and bone uptake were influenced by carrier, and the relationship between changes in HA binding and carrier perrhenate concentration appeared to be related to radiochemical purity. However, the results of animal studies suggested than bone accumulation 188Re-HEDP with adequate radiochemical purity and HA uptake did not necessarily predict acceptable biodistribution of 188Re-HEDP.
12.5.1.3 Other Rhenium-186/188 (186Re/188Re) Analogs
In general, most Re(V) phosphonate complexes which have been prepared and evaluated show good stability and high skeletal targeting and low soft tissue uptake and must be evaluated further to develop any compelling justification that they would be superior to Re(V) HEDP. Other rhenium analogs which have been prepared and evaluated for bone uptake include the pharmacologic bone pain palliation agent, 1-hydroxy-3-aminopropylidenediphosphonate (APD; Fig. 12.6). In one study, the 186Re-labeled phosphonate with 186Re-ADP (Alyafei et al. 1999) was prepared by tin chloride reduction of 186Re-perhenate in the presence of gentisic acid. Studies in normal mice 1–240 h post injection demonstrated high bone/blood ratios ranging from 25 to 189 over the evaluation period, with low soft tissue accumulation. In similar studies, generator-derived 188Re-potassium perrhenate was reduced at acidic pH with tin fluoride in the presence of the ADP ligand to provide the 188Re-ADP product which was subsequently evaluated in rats (Arteaga de Murphy et al. 2001). Biodistribution data and skeletal targeting were similar to 99mTc-ADP, suggesting to the authors that the 188Re is a promising agent for further evaluation. Rhenium-188 has also been complexed in >95 % radiochemical yield with N,N,N’,N’-tetrakis-methylene phosphonic acid (EDTMP; Fig. 12.6) at low pH with stannous chloride (Oh et al. 2002) and compared with 188Re-HEDP in Wistar male rat biodistribution studies. The data were similar for both agents, with rapid clearance and low soft tissue uptake and high bone uptake. With regard to complex structures, none of these agents were evaluated so the comparison with the complex oligomeric structure of Re(V)-HEDP is unknown.
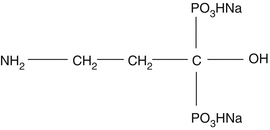

Fig. 12.6
Structure of sodium salt of ADP
As the phosphonate groups in 186/188Re-HEDP are used as both ligands for coordination and as carrier to HA in bone, there is a possibility that it may decrease the inherent affinity of HEDP for bone. In order to circumvent such possibilities, 186Re-monoaminemonoamidedithiol (MAMA)- and 186Re-mercaptoacetylglycylglycylglycine (MAG3)-conjugated bisphosphonate compounds (186Re-MAMA-BP,186Re-MAMA-HBP, and 186Re-MAG3-HBP) have been developed (Ogawa et al. 2004, 2005, 2006). Their chemical structures have been illustrated in Fig. 12.7.
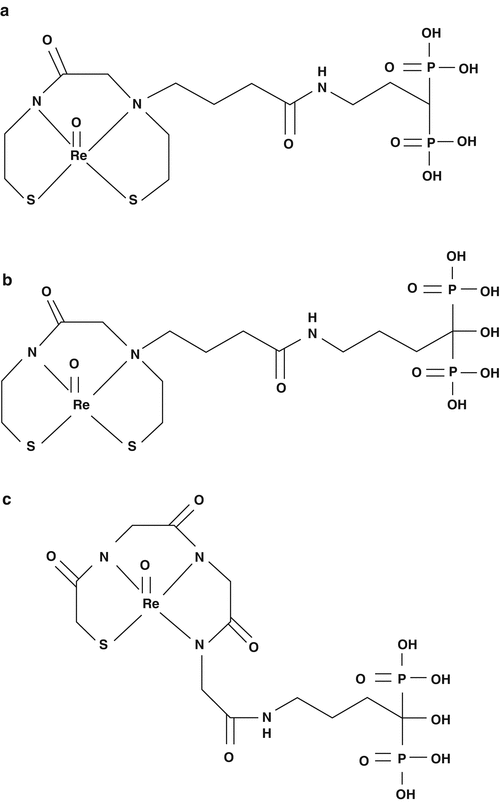

Fig. 12.7
Chemical structures of (a) 186Re-MAMA-BP, (b) 186Re-MAMA-HBP, (c) 186Re-MAG3-HBP
On a similar theme Uehara et al. (2007) developed a tricarbonyl [186Re][(cyclopentadienylcarbonyl amino)-acetic acid] rhenium complex ([186Re]CpTR-Gly)-conjugated bisphosphonate [186Re-CpTR-Gly-APD] which has been reported to have characteristics superior to those of 186Re-HEDP, such as higher stability in plasma, a higher binding rate for HA, higher bone accumulation, and lower plasma protein binding. Subsequently De Rosales et al. (2010) have developed 188Re(CO)3-DPA-alendronate which showed higher stability in vitro compared with 188Re-HEDP and superior biodistribution of radioactivity than 188Re-HEDP. Chemical structures of [186Re]CpTR-Gly-APD and 188Re(CO)3-DPA-alendronate are shown in Fig. 12.8.
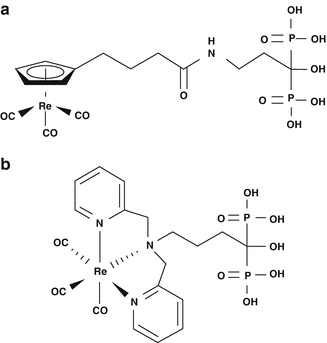

Fig. 12.8
Chemical structures of (a) [186Re]CpTR-Gly-APD, and (b) 188Re(CO)3-DPA-alendronate
12.5.2 Lutetium-177 Diphosphonates
More recently, several studies have described the use of 177Lu-labeled phosphonate complexes for bone pain palliation. Lutetium-177 is a lanthanide (+3) therapeutic radioisotopes of great interest (Banerjee et al. 2015), since it can be reactor-produced even at moderate thermal neutron flux with very high specific activity (Dash et al. 2015). Ando et al. have reported for the first time the utility of 177Lu-ethlyendiammine tetramethylene phosphonate (EDTMP; Fig. 12.9) (Ando et al. 1998). In one study, the International Atomic Energy Agency (IAEA) sponsored a multicenter trial evaluating the efficacy of 177Lu-ethlyendiamminetetramethylenephosphonate (EDTMP) (Chopra 2011).
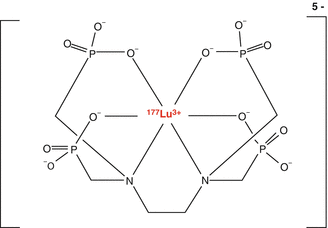

Fig. 12.9
177Lu-ethlyendiamminetetramethylenephosphonate (EDTMP).
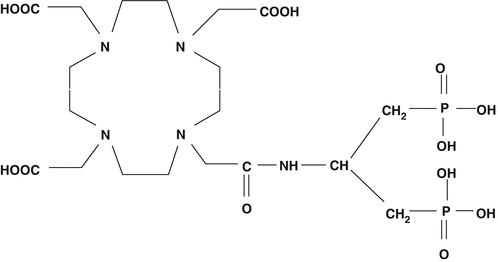
In addition, the 177Lu-labeled (DOTMP) has also been evaluated. The 177Lu-labeled (4-{[bis-(phosphonomethyl) carbamoyl} methyl}-7,10-bis (carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl)acetic acid (BPAMD; Fig. 12.10) has been developed as a new bone pain palliation agent (Fellner et al. 2010). The +3 DOTA is an excellent lanthanide-chelating moiety which has the specific advantages of very tightly binding +3 metal and allowing very facile nearly quantitative radiolabeling yields of lanthanides under mild conditions with the formation of a highly stable Lu complex. Initial human trials have demonstrated the excellent targeting of this agent in skeletal lesions (Fellner et al. 2010) and MIRD/OLINDA/EXM dosimetry estimates utilizing data from 8 patients with skeletal metastases from prostate cancer have also been recently reported (Schuchart et al. 2013; Yousefnia et al. 2015). These studies have demonstrated very rapid blood clearance whole-body biexponential clearance with a second half-life of 62 ± 19 h and a metastases site clearance of 57–162 h. In addition, the broad interest in the production and use of 177Lu (Pillai and Knapp 2015)—currently focused on the use of therapeutic peptides such as 177Lu-DOTATATE and DOTATOC—would suggest that the expected broad availability of pharmaceutical grade 177Lu may catalyze further interest and broad use of 177Lu-BPAMD.