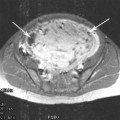
Fig. 1
Sagittal T2-weighted MRI of diffuse (a) and focal (b) adenomyosis
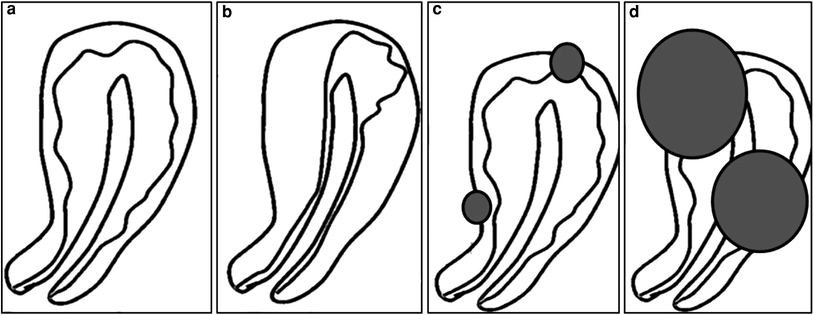
Fig. 2
Different types of adenomyosis with or without fibroids. a Diffuse pure adenomyosis; b focal pure adenomyosis (adenomyoma); c diffuse adenomyosis dominance with fibroids; d diffuse adenomyosis with fibroid dominance
The clinical diagnosis is challenging, as the presenting symptoms overlap with common uterine disorders such as fibroids of the uterus (Azziz 1989; Vercellini et al. 1993). Adenomyosis is often underdiagnosed and is responsible for disabling symptoms such as in particular heavy menstrual bleeding and pain, with or without bulk related symptoms and fertility issues in premenopausal women (Valentini et al. 2011).
The reported occurrence of adenomyosis varies significantly. The prevalence of adenomyosis in tissues obtained from hysterectomy is reported between 8.8 and 31 % (Benson and Sneeden 1958; Owolabi and Strickler 1977). With broad criteria for the diagnosis of adenomyosis, a prevalence as high as 70 % in women between 40 and 50 years of age is suggested (Azziz 1989). The incidence of adenomyosis in the population at risk is between 8.1 and 16.7 % with clinical manifestations in about two-thirds of women. Of women with clinical manifestations of adenomyosis, about one-fifth are under 40, but the vast majority are between 40 and 50 years. (Utsunomiya et al. 2004; Bergeron et al. 2006; Peric and Fraser 2006). Risk factors for adenomyosis are presumed to be related to reproductive activity (with an increased risk in multiparity, miscarriage, and endometriosis), smoking, Caesarean section, induced abortion or curettage (Vercellini et al. 2006).
2 Diagnostic Imaging and Fundamental Radiological Signs
In women with suspected adenomyosis, the first-line imaging technique is usually transvaginal ultrasound (TVUS). TVUS is inexpensive and readily available. Fundamental TVUS signs are (1) increased myometrial echogenicity or linear hyperechoic bands extending deep into the myometrium, indicating the presence of islets of ectopic endometrial tissue, (2) hypoechoic areas in the myometrium compatible with hyperplasia of the muscle tissue surrounding the ectopic tissue, (3) anechoic areas due to glandular dilatation or myometrial cysts, (4) poor definition of the junction zone, and (5) enlargement of the uterus with asymmetrical thickening of one of the walls. The presence of at least three of these signs is highly suggestive of adenomyosis (Dueholm 2006). Sensitivity, specificity and accuracy of TVUS for adenomyosis vary among 80–86, 50–96 and 68–86 %, respectively (Reinhold et al. 1999).
Magnetic resonance imaging (MRI) is particularly useful both in doubtful TVUS cases and in providing a complete evaluation of the disease with its panoramic views. With T2-weighted images and contrast enhanced T1-weighted MRI, the thickness of the junction zone can reliably be measured; a thickness over 12 mm is considered diagnostic for adenomyosis. The presence of foci of high signal intensity within the myometrium constitutes an additional, but not a mandatory criterion (Figs. 1, 3). MRI is a reliable modality for diagnosing adenomyosis, with a sensitivity varying in the literature between 78 and 88 % versus 53 and 89 % for TVUS and a specificity of 67–100 % versus 67–98 % for TVUS (Reinhold et al. 1999; Tamai et al. 2005). MRI can categorise adenomyosis as focal or diffuse and can be repeated in time to evaluate the effect of treatment.
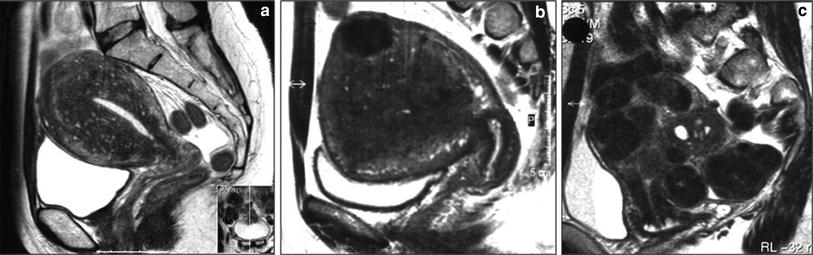

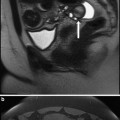
Fig. 3
Sagittal T2-weighted MRI of different types of adenomyosis. a Diffuse pure adenomyosis; b adenomyosis dominance with fibroid; c adenomyosis with fibroid dominance
Three different groups of uterine adenomyosis are easily identified with MRI: (1) pure adenomyosis, (2) adenomyosis with fibroid predominance, and (3) uterine fibroids with adenomyosis predominance (Fig. 2). Adenomyosis may be subdivided in diffuse or focal. Focal adenomyosis is also known as adenomyoma. From personal experience, maybe around 80 % of these women have adenomyosis mixed with fibroids, 15 % pure diffuse adenomyosis and 5 % pure focal adenomyosis (adenomyoma).
Adenomyosis with fibroid predominance is defined when fibroids are larger than 5 cm with extensive contact with the uterine cavity (two-thirds of the cavital surface area) and patients presented with dominant bulk symptoms in the presence of adenomyosis. If the combined fibroids were smaller than 5 cm in size and/or covered less than two-thirds of the cavital surface area, these cases were defined as combined disease of predominant adenomyosis (Froeling et al. 2011).
Regarding follow-up after uterine artery embolisation (UAE) for adenomyosis, evolution of symptoms is the most important parameter. In patients with improvement of symptoms and satisfaction with treatment, MRI follow-up may not be necessary as a routine procedure. However, in patients with insufficient clinical response after UAE, MRI can be helpful to compare thickness of the junction zone, the infarction rate, the uterine volume, and other parameters with baseline findings and thus MRI follow-up may guide clinical decision making.
3 Treatment Options
3.1 Medical Treatment
Medical treatment of adenomyosis ranges from local treatment with the release of medications by a intrauterine device (IUD) to systemically administered treatment.
IUD-released progestogens are used to reduce heavy menstrual bleedings in women with adenomyosis. These medications cause the decidualisation of the endometrium and consequently atrophic changes to reduce the amount of bleeding as in women with symptomatic adenomyosis (Fedele et al. 1997).
Medications available for systemic administration include gonadotropin-releasing hormone (GnRH) agonists. Adenomyosis is an oestrogen-dependent disease. GnRH agonists induce menopause by reducing the release of pituitary gonadotropins. The levels of oestrogen are lowered, producing atrophy and reduced volume of the uterus. If the therapy is interrupted, the effect is reversible (Faquhar and Brosens 2006).
3.2 Surgery
Excision or enucleation is usually the preferred surgical approach for focal adenomyosis, but the type of treatment is heavily dependent on the type of lesion and the extent of myometrial involvement (Faquhar and Brosens 2006). Hysterectomy is the preferred surgical option for women with symptomatic adenomyosis if there is deep involvement of the myometrium. In cases of diffuse adenomyosis in which hysterectomy is considered, trans-vaginal access is preferable to trans-abdominal access due to the lower morbidity and shorter hospital stay of the former. Regarding the complications of trans-vaginal hysterectomy, a study on two groups of patients, one with fibroids and the other with adenomyosis reported a higher risk of lesions to the urinary bladder in patients from the latter group (Furuhashy et al. 1998). The reason is probably the greater complexity in identifying the vesico-vaginal septum due to the presence of adhesions. This is explained by the fact that adenomyosis and endometriosis may coexist in the same patient with pelvic adherences very frequently present in the latter (Valentini et al. 2011).
Hysterectomy is usually indicated as a definitive treatment. Rates of complication after hysterectomy range between 1.5 and 29.3 %. Hysterectomy is associated with complications such as blood loss, bowel and general uro-genital injury, pain, and infection. Recovery time is reported to range between 6 and 8 weeks (Faquhar and Brosens 2006; Meyers and Steege 1998; Dembek et al. 2007), and health care-related expenses and lost time at work (Volkers et al. 2008) render hysterectomy an option associated with high costs.
3.3 MRI-Guided ‘High-Intensity Focused Ultrasound’
MR-HIFU is a new, image-guided, non-invasive technique which enables treatment of tumours by thermoablation by ultrasound waves. The treatment is completely guided by MRI, which offers advantages for therapy planning, monitoring and visualisation of the treatment result. MR-HIFU has a broad spectrum of applications, including ablation of uterine fibroids (Voogt et al. 2011). Most of these applications are still under research. The advantage of the non-invasive character of the treatment is that it can be performed on an outpatient basis and that recovery is fast. MR-HIFU has been used in adenomyosis with varying results. Therefore, although the technique seems to be a useful alternative, further studies are needed to clarify its effective role (Rabinovici and Stewart 2006).
3.4 Embolization
In 1995, Ravina published the first report of women treated by uterine artery embolization (UAE) for symptomatic uterine fibroids (Ravina et al. 1995). UAE has emerged as an effective therapy in the treatment of uterine fibroids. The clinical success rate of UAE for uterine fibroids with respect to symptomatic improvement of associated menorrhagia and pelvic pain ranges from 85–95 % to 80–90 % (Katsumori et al. 2007; Goodwin and Spies 2009). There is about 25 % chance of failure of symptom control or recurrence after UAE for uterine fibroids at a 5 year follow-up (Spies et al. 2005a, b; Lohle et al. 2008). This minimally invasive therapeutic alternative to surgery has been reported to be associated with high patient satisfaction rates (Goodwin et al. 1997; Worthington-Kirsch et al. 1998; Spies et al. 2002; Goodwin et al. 2008; Hehenkamp et al. 2008; Hirst et al. 2008).
Based on the similarity of symptoms caused by uterine fibroids and adenomyosis and the positive results after UAE for fibroids, this interventional procedure has been investigated as a possible option to treat adenomyosis. Successful infarction of symptomatic fibroids with UAE may also be achievable in women suffering from focal or diffuse adenomyosis with or without fibroids.
4 Embolization in Adenomyosis
Although clinical manifestations of uterine fibroids and uterine adenomyosis are similar, their treatment may differ. In addition to medical treatment, radical, or conservative surgery is well-established treatment options for symptomatic fibroids (Dumousset et al. 2008). Adversely, uterine adenomyosis usually requires hysterectomy because of poor results of hormone treatment or endometrial ablation (McCausland and McCausland 1998).
Embolization of the uterine artery is a therapeutic approach adopted for treating fibroids. Adenomyosis and uterine fibroids may coexist and symptoms can be similar. The interventional treatment accepted for fibroids might therefore be applicable to adenomyosis. Several studies have documented treatment of adenomyosis with UAE (Smith et al. 1999; Goodwin et al. 1999; Spies et al. 1999; Wood 2001; Siskin et al. 2001; Chen et al. 2002; Jha et al. 2003; Toh et al. 2003; Kim et al. 2004; Pelage et al. 2005; Kitamura et al. 2006; Kim et al. 2007; Lohle et al. 2007; Duan et al. 2008; Bratby and Walker 2009; Froeling et al. 2011). Although the first results of UAE for adenomyosis were disappointing, later studies showed substantial clinical improvement in the majority of treated women with adenomyosis. Similar to UAE in fibroids, the targeted embolization with occlusion of uterine artery vessel branches with embolic material will induce cessation of arterial blood flow to the adenomatous tissue. Intentional infarction will eventually result in complete or partial elimination of adenomyotic foci and subsequently relief of symptoms.
4.1 Uterine Artery Embolization Procedure
In 1995, the first report was published of women treated by UAE for symptomatic uterine fibroids (Ravina et al. 1995). The UAE catheterisation technique for symptomatic adenomyosis is no different from the technique for symptomatic fibroids, apart may be from the angiographic embolization endpoint and maybe the embolic agent particle size. It is reported by some authors that the angiographic embolization endpoint should be when there is complete stasis at the level of the ascending distal part of both uterine arteries in case of adenomyosis (Lohle et al. 2007; Smeets et al. 2011). Embolization is performed by using a particulate embolic agent. The most widely used embolic agent is non-spherical polyvinyl alcohol particles ranging from 255 to 900 microns in size (Popovic et al. 2011). Others prefer the use of spherical embolics, calibrated microspheres, ranged in size from 500 to 700 microns, in order to facilitate deep penetration into the small afferent arterioles of adenomyosis. In contrast to the perifibroid vascularization pattern of uterine fibroids, adenomyosis has a deep and more diffuse distribution of parallel afferent arterioles throughout the myometrium (Dundr et al.2006). The difference in vascularisation might explain the reported higher failure rate after UAE in women with adenomyosis compared with UAE for fibroids. The currently available data do not seem to indicate a preferred embolic agent for use in women with symptomatic adenomyosis. Although in part based on speculation, to my experience, deep penetration with the embolic agent seems to be needed for optimal infarction of areas with adenomyosis. Calibrated microspheres are able to selectively occlude the tiny arterial branches of the adenomatous tissue deep in the uterine stroma and thus create adequate tissue infarction (Fig. 4). It has been demonstrated that calibrated microspheres have a predictable behaviour. Their uniform size and shape, their constant compressibility and elasticity results in deeper penetration into the small arterioles than non-spherical polyvinyl alcohol particles (Chua et al. 2005; Lohle et al. 2007; Smeets et al. 2011).
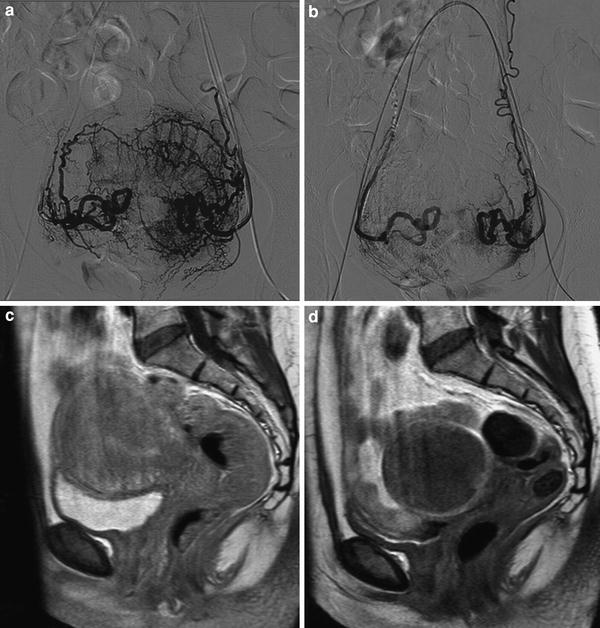

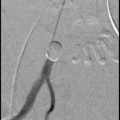
Fig. 4
UAE in a woman with pure adenomyosis. a Frontal angiogram with contrast injection of both uterine arteries demonstrating the vascularization of adenomyosis with deep penetrating parallel arterioles; b Frontal angiogram after UAE with the proper embolization end-point; c Sagittal contrast enhanced T1 weighted MRI of pure adenomyosis before UAE; d Sagittal contrast enhanced T1 weighted MRI 3 months after UAE demonstrating complete infarction of pure adenomyosis with uterine volume reduction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree