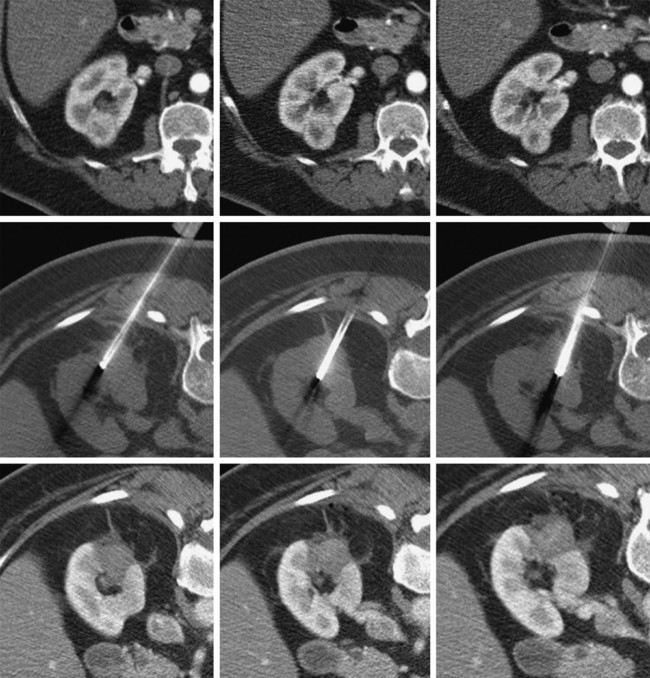
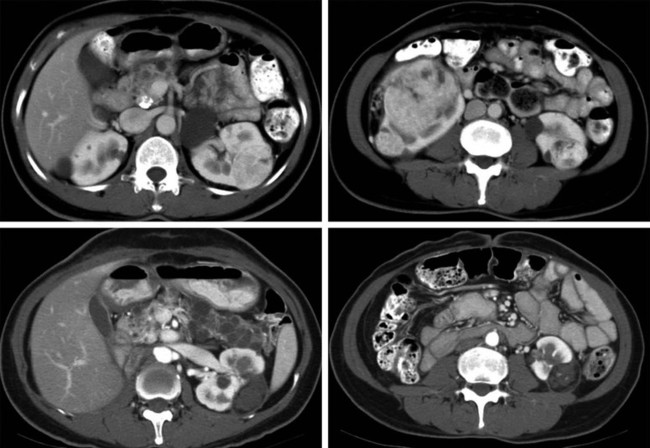
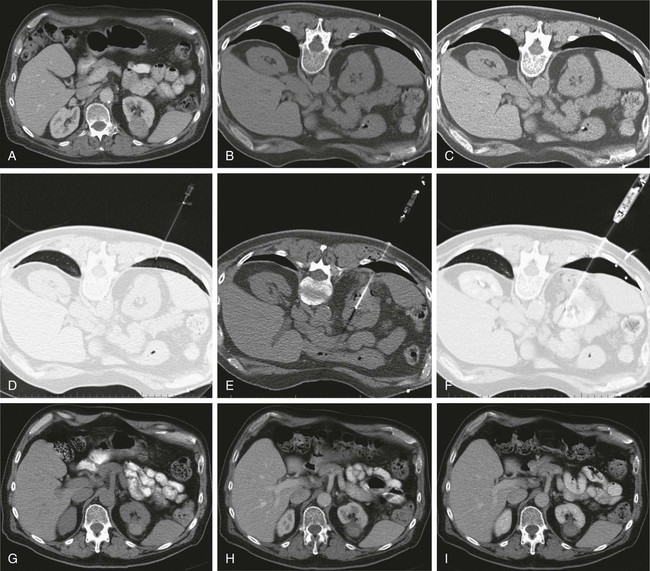
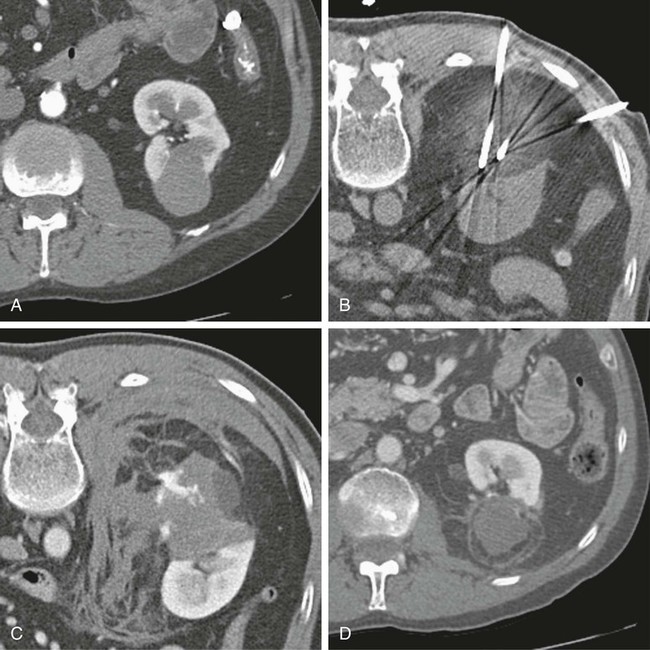
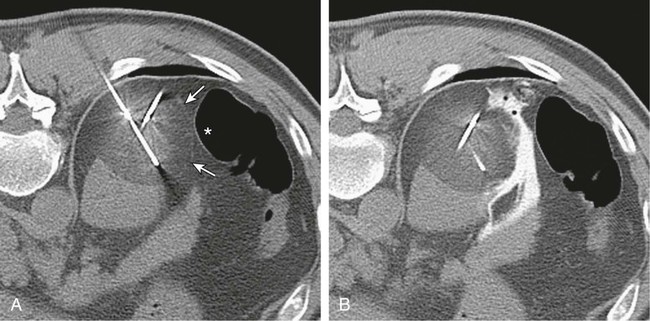
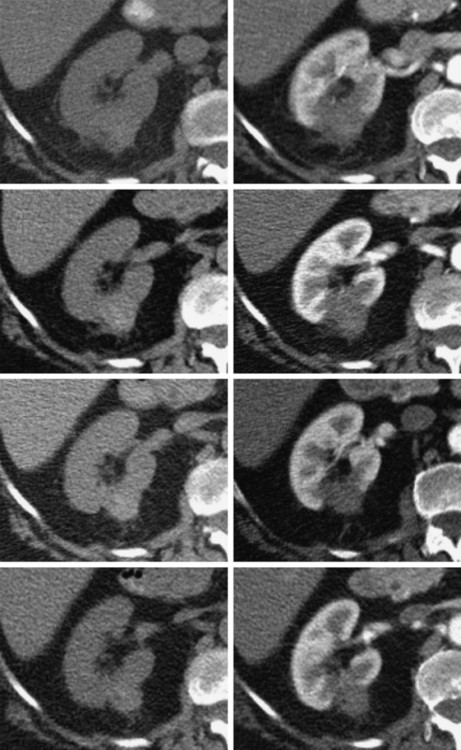
In 2013, over 65,150 new cases of kidney cancer were diagnosed in the United States, resulting in over 13,680 deaths.1 Renal parenchymal tumors (i.e., renal cell carcinoma [RCC]) account for the majority (85%) of kidney cancers.2 Urothelial cancers of the renal pelvis (i.e., transitional cell carcinoma [TCC]) account for most of the remaining cases. The incidence of RCC has increased on average 2% to 3% a year for the past 3 decades.3 At present, over 50% of RCCs are detected incidentally during imaging studies prompted by nonurologic complaints.4,5 Tumors detected incidentally are smaller (stage I [Table 148-1]) at diagnosis and are often asymptomatic.3 Partial nephrectomy is the standard of care for small RCCs and provides excellent results. Recurrence-free survival, cancer-specific survival, and overall survival rates for open or laparoscopic partial nephrectomy have been reported as better than 95%, 97%, and 89%, respectively.6 Other alternative treatment options advocated by the American Urological Association (AUA) include active surveillance and thermal ablative therapies.6 TABLE 148-1 American Joint Committee on Cancer TNM Staging of Renal Cell Carcinoma T1: Tumor is 7 cm or less in greatest dimension and is limited to the kidney. T1b: Tumor is greater than 4 cm but less than 7 cm. T2: Tumor is more than 7 cm in greatest dimension and is limited to the kidney. T3: Tumor extends into major veins or invades the adrenal gland, sinus fat, or perinephric fat, but does not extend beyond Gerota fascia. T3a: Tumor invades the adrenal gland. T3b: Tumor grossly extends into the renal vein or vena cava below the diaphragm. T3c: Tumor grossly extends into the vena cava above the diaphragm. From Edge SB, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2009. Thermal ablative therapies destroy tumors by using thermal energy—either heat (e.g., radiofrequency [RF], laser, microwave, or focused ultrasound) or cold (e.g., cryoablation).7 The technologies most widely used in clinical practice for ablation of renal tumors are RF ablation (RFA) (Figs. 148-1 to 148-4) and cryoablation (Figs 148-5 and 148-6). Laser, microwave, and high-intensity focused ultrasound technologies are in various stages of experimental or early clinical application but have not been utilized to any substantial degree in clinical practice for treating renal tumors. With either cryoablation or RFA, once the tumor is ablated, it is left in situ. This is a departure from the basic oncologic surgical principle of excision with clear margins. For ablation technology to be considered a clinically acceptable alternative to extirpation, it must be reliable and completely destroy all viable tumor. There must also be a means of imaging and monitoring the area of ablation to preserve and protect surrounding vital structures while ensuring complete tumor destruction. Thermal ablation of renal tumors can be performed either intraoperatively or percutaneously. Historically, RFA has been used most commonly for percutaneous image-guided ablation, whereas cryoablation has been used most commonly in open or laparoscopic approaches.8 More recently, small-diameter cryoprobes have become available for percutaneous use, resulting in an increasing number of percutaneous cryoablation procedures. At the same time, more urologic surgeons have adopted intraoperative RFA over cryoablation, resulting in an increasing number of laparoscopic RFA procedures. Currently, three RFA devices have been approved by the U.S. Food and Drug Administration (FDA) and are commercially available in the United States. Each of these uses a generator to deliver alternating electric current via an electrode, causing ionic agitation and frictional heating within the target tissue. Each of these ablation systems uses a different strategy to obtain the largest possible zone of ablation. The maximum attainable in vivo ablation zone varies by applicator size and design and is consistently less than the ex vivo maximum,9 in part owing to the presence of adjacent flowing blood or large fluid-filled structures that produce a cooling or heat sink effect. The two cryoablation systems currently available in the United States for percutaneous image-guided ablation are the SeedNet System (Galil Medical, Arden Hills, Minn.) and Endocare (HealthTronics, Austin, Tex.). In both systems, highly compressed argon gas is allowed to expand in a Joules-Thompson chamber in the distal end of the cryoprobe, resulting in intense cooling that creates an ice ball within the target tissue. Helium is used in a similar fashion to actively thaw the ice ball. Cryoprobes used in percutaneous ablation range in size from 17 gauge to 2.4 mm in diameter. The critical temperature required to achieve tissue necrosis is −19.4°C in normal renal tissue10 and −40°C in cancer cells.11 This temperature must be transmitted throughout the entire tumor. There is a rapid drop in temperature at the edge of the ice ball, so to achieve the required temperature for cell death throughout the entire tumor, the ice ball must extend at least 3.1 mm beyond the tumor margin.12 Thermal ablation is an alternative to partial nephrectomy in patients who may not be good surgical candidates, have multiple RCCs, have limited renal function, or refuse surgical intervention.13 The majority of incidentally detected small renal tumors are diagnosed in older patients who may have other medical comorbidities (e.g., cardiovascular or respiratory conditions) that result in an unacceptably high operative risk (see Fig. 148-1). In patients with renal insufficiency and those with only one anatomic or functioning kidney, preservation of renal function is of utmost importance.14 Thermal ablative therapies are superior to partial nephrectomy in preserving renal function, so ablative therapy should be considered for patients with limited renal function, because it may help minimize the need for dialysis in the future. When residual or recurrent disease is identified after nephron-sparing surgery or ablation, thermal ablation should be considered as the least invasive therapy. Patients with von Hippel-Lindau disease, hereditary papillary cell carcinoma, or hereditary clear cell carcinoma have a genetic predisposition for RCC. Many of these patients will ultimately require nephrectomy, but ablative therapy may prolong the time to resection.15 Patients with multiple synchronous RCCs (sporadic or genetic) may be treated with surgical resection of the larger lesions and ablation of the smaller lesions (see Fig. 148-3). For the most part, thermal ablation of renal tumors is indicated for patients with tumors confined to the kidney. As such, metastatic disease, local invasion, nodal involvement, and extension into the renal vein or inferior vena cava are relative contraindications to ablative therapy. On the other hand, when isolated metastases are amenable to surgical or ablative therapy, ablation of the primary tumor can provide durable local control.16 When RCC causes hematuria, embolization should be considered first-line therapy; however, utilizing RFA for palliation of intractable hematuria has also been reported.17,18 Tumor size and location should be carefully considered prior to choosing ablation therapy. Most published series indicate that complete ablation can be more easily achieved for smaller tumors. In general, an ideal tumor for percutaneous RFA is smaller than 4 cm.19,20 As the tumor size increases, the technical success rate decreases. In a study of 125 RCCs, for each 1-cm increase in tumor diameter beyond 3.6 cm, the likelihood of tumor-free survival decreased by a factor of 2.19.20 Furthermore, when RCC grows outward and does not abut or invade the collecting system, thermal ablation can be more easily achieved.19,21 Gervais et al. described a classification scheme for renal tumors with respect to their location.22 In their system, tumors with at least 25% of their volume extending beyond the renal contour, with no tumor extending into or adjacent to the renal sinus, are classified as exophytic. RCCs that are entirely confined to the cortex without any contact with or invasion of the renal sinus are classified as intraparenchymal. Tumors that grow internally into the renal sinus are classified as central. And finally, when tumors have both exophytic and central components, they are classified as mixed tumors. From a practical standpoint, the exophytic and intraparenchymal tumors can be considered “non-central” tumors, and similarly, the central and mixed tumors can be considered “central” tumors. Satisfactory short-term results have been reported with ablation of large tumors (>4 cm) and central tumors, but more than one ablation session may be necessary, and hemorrhagic complications may be more common in these cases.19,21,23 All patients should undergo a preprocedural assessment that includes serum creatinine level, estimated glomerular filtration rate (GFR), platelet count, and a coagulation profile. Patients with proven RCC should have chest imaging (e.g., chest radiograph) and a metabolic profile. These are standard basic tests for surveillance of metastatic disease, the incidence of which is very low but still possible for small tumors. Patients also should be assessed for their ability to tolerate intravenous sedation and should meet institutional criteria. Local institutional factors and physician preferences determine whether intravenous sedation19 or general anesthesia24,25 is used during ablation therapy. General anesthesia optimizes patient tolerance, allows greater control of respiratory motion when the applicators are being placed, and may facilitate accurate tumor targeting. A percutaneous biopsy is recommended prior to ablation of renal tumors.26 The differential diagnosis of a small enhancing renal mass includes benign entities such as lipid-poor angiomyolipoma, oncocytoma, papillary adenoma, and metanephric adenoma. As the size of a renal mass decreases, the likelihood of a benign diagnosis increases. Approximately 25% of renal tumors smaller than 4 cm are benign, but when tumors smaller than 1 cm were resected, over 40% were benign.27 About 5% of angiomyolipomas are indistinguishable from small RCCs on cross-sectional imaging.28 Tuncali et al. reviewed biopsy and imaging data of 27 patients referred for cryoablation of a small renal mass.29 Ten lesions (37%) smaller than 2 cm were deemed benign. The “diagnostic accuracy” of renal biopsy has improved over the years, particularly with the addition of routine core biopsies, and is better than 95% in most contemporary series. Sensitivity for detection of malignancy ranges from 84% to 100% in studies of renal masses published after 2006.30 When percutaneous biopsy establishes a diagnosis (e.g., lymphoma or angiomyolipoma) other than RCC, ablation is not indicated. A positive result with diagnosis of RCC provides details about tumor subtype and grade, information that may become relevant during follow-up visits, insurance claims, or should the patient ever develop metastatic disease requiring systemic therapy. A positive result is also important for the validation of ablation therapy and defining the standard of care for small renal masses in the future. On the other hand, concerns over false-negative biopsy results remain; some clinicians recommend proceeding with therapy when a negative histologic result conflicts with imaging findings.31,32 Ideally, the biopsy should be performed during a separate encounter so sufficient time is given for a complete histologic evaluation. For large tumors (>4 cm), consideration may be given to selective embolization prior to ablation. Embolization may help improve the effectiveness of ablation by reducing perfusion-mediated cooling (the “heat sink effect” during RFA) or heating (during cryoablation) and may help reduce the risk of hemorrhagic complications.33–35 Ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) have been used to guide renal ablation proceures.25,36–38 Ultrasonography provides valuable real-time multiplanar imaging for initial tumor targeting and placement of the applicators. Some investigators have reported favorable outcomes for ultrasound-guided RFA compared with other imaging modalities.39 However, inability to visualize the tumor, gas bubbles during RFA, and large tumor size limit the effectiveness of ultrasonography.40 During cryoablation procedures, the edge of the ice ball creates intense acoustic shadowing that precludes assessment of the leading edge of the ablation zone.41–43 Ultrasonography is not optimal for assessment of nearby loops of bowel, and images may become degraded by perinephric hematoma that may develop during the procedure. Currently, CT is the imaging modality most widely used for percutaneous image-guided renal ablation.19,24,25 It provides guidance for precise probe placement and is not affected by gas formation during RFA. CT is ideal for monitoring other vital structures (e.g., bowel) that may come in contact with the tumor. During cryoablation, the location and size of the ice ball can be visualized rather clearly on CT (see Fig. 148-6),44 whereas in RFA the ablation zone is less clearly visualized, and the boundary between treated and untreated tissue is not precisely demarcated. To overcome this problem, a contrast-enhanced postablation CT examination is typically performed just before completing the RFA (see Fig. 148-1).24,25 CT fluoroscopy enables real-time visualization of the applicator tip as it is being placed and facilitates precise targeting of the tumor. The main disadvantage of CT or CT fluoroscopy is patient and operator exposure to ionizing radiation. For this reason, some researchers advocate using a combination of ultrasonography and CT. Ultrasonography can be used for initial placement of the applicators, and CT can be used for any additional imaging during the procedure, including repositioning the electrodes, monitoring adjacent loops of bowel, assessing the size and margins of the ice ball, and performing a contrast-enhanced study to confirm complete tumor ablation. MRI is an ideal imaging modality for ablation guidance and monitoring because it provides excellent soft-tissue contrast and spatial resolution compared with CT and ultrasound.37,38,45 In addition to providing multiplanar and near real-time imaging, MRI also allows for monitoring of tissue coagulation and treatment results,37 which CT does not. Also, the lack of ionizing radiation is a significant advantage over CT. Unfortunately, current challenges in producing special MRI-compatible equipment and the lack of availability of interventional MRI units have limited its use in daily clinical practice. Optimal patient positioning is determined during the planning phase when cross-sectional images are reviewed to assess the feasibility and safety of access to the target tumor. In a typical ablation procedure, the patient is placed in the prone position. During CT-guided procedures, it is helpful to move the arms away from the body in an abducted and extended position and place them next to the patient’s head (“the superman position”). This reduces streak artifact and facilitates tumor visualization. When patients are under general anesthesia, care must be taken to not overextend the arms and to provide adequate padding to avoid brachial plexus injury.46 An ipsilateral decubitus position can help reduce the risk of pneumothorax, although it may limit access to the tumor for insertion of the applicators. A contralateral decubitus position can help separate loops of bowel from a tumor in the anterior or lateral aspect of the kidney. In rare instances, anteriorly or laterally located tumors may be treated with the patient in the supine position. An ablation margin of 5 to 10 mm around the tumor is desirable.12 The size of the ablation zone depends on the size and vascularity of the lesion, the lesion’s proximity to vascular structures, the ablation modality used, and the number, size, and configuration of the applicators. Care must be taken to avoid adjacent vital structures such as bowel and large vessels, and consideration must be given to both the potential applicator path and the projected zone of ablation. When vital structures are detected in the path of the applicator or are contiguous with the expected ablation zone, simple and noninvasive measures may suffice to render the procedure safe. These maneuvers include changing the patient’s position or levering the applicator against the skin to lift the tumor off the bowel or vascular structure. Torquing the applicator can potentially increase the distance from the tumor to the bowel by 3 or 4 mm.47 Ideally, a safe margin between the probe tines and the nearest adjacent bowel is 1 to 2 cm.48 To protect nearby structures such as bowel, nerves, or muscles, gas insufflation or hydrodissection (see Fig. 148-6) can be used to create a barrier for heat transmission.19,49,50 During RFA procedures, nonionic sterile fluid may be instilled using a small-gauge needle placed between the tumor and the bowel under CT or MRI guidance. With injection of 135 to 150 mL of fluid, one can displace the adjacent bowel loop by 2.1 to 2.5 cm.50 Often, additional hydrodissection attempts are necessary because the fluid spills into the paracolic gutters or Morrison pouch.50 Alternatively, gas can be insufflated into the peritoneal cavity or perinephric space to prevent thermal damage to adjacent vital structures. Gas has a tendency to dissipate throughout the peritoneal space; thus, larger volumes of gas are required compared with fluid. When injected into the peritoneum, a gas volume of 1200 mL yields 1.5 cm of bowel displacement.51 When injected into the perirenal space, 15 to 20 mL of gas may be sufficient to achieve a similar degree of bowel separation.52 Adequacy of insufflation is best monitored with CT because gas can obscure the view of the tumor when MRI or ultrasonography is used.51 If hydrodissection does not create the desired effect, large angioplasty or esophageal dilation balloons may be inserted between the tumor and the structure at risk.48 More than one balloon may be necessary to create an adequate separation between the ablation zone and adjacent structures. Each balloon may be placed in coaxial fashion through an introducer sheath. Initially, a needle is inserted into the desired space. Over a guidewire, an introducer sheath is advanced into the desired position. The balloon is then placed into the sheath but not beyond it. With the balloon in position, the sheath is withdrawn. Balloon expansion is completed once the optimal position has been obtained. Peripheral thermosensors can be placed to ensure adequate ablation and to prevent thermal injury to normal renal parenchyma and adjacent structures (e.g., periureteric tissue). Carey et al. reported 100% primary effectiveness for RFA of 37 tumors that were 3-5 cm in diameter in which real-time temperature feedback of the ablation zone was used to determine the appropriate treatment endpoint.53 Ablation was continued until both deep and peripheral thermosensors recorded temperatures of at least 60°C. These independent real-time thermosensors can also be used to determine whether and where an electrode has to be redeployed. Thermal damage to the ureter or ureteropelvic junction (UPJ) can lead to stricture formation. To reduce this risk during RFA of an adjacent renal mass, retrograde pyeloperfusion with a cooled nonionic solution can be performed.54–56
Thermal Ablation of Renal Cell Carcinoma
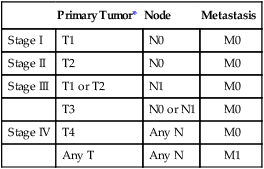
Primary Tumor*
Node
Metastasis
Stage I
T1
N0
M0
Stage II
T2
N0
M0
Stage III
T1 or T2
N1
M0
T3
N0 or N1
M0
Stage IV
T4
Any N
M0
Any T
Any N
M1
Ablation Devices
Radiofrequency Ablation
Cryoablation
Indications
Patient Selection
Tumor Selection
Technique
Patient Assessment
Imaging Modalities
Ablation Procedure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thermal Ablation of Renal Cell Carcinoma