LEARNING OBJECTIVES
1. Describe the anatomy of the thoracic aorta.
2. State the normal dimensions of the thoracic aorta.
3. Describe the morphology and etiology of thoracic aortic aneurysms.
4. Describe the imaging appearance of the following aortic pathologies (and their complications) associated with the acute aortic syndrome:
• Penetrating atherosclerotic ulcer
• Intramural hematoma
• Acute aortic dissection
5. Recognize the normal appearance and potential complications of both surgical and endovascular aortic repair.
ANATOMY OF THE THORACIC AORTA
The aortic root is defined by three components (Fig. 19.1): (i) the aortic annulus (virtual basal ring), which is an ovoid perimeter defined by the inferior attachment of the aortic valve leaflets, (ii) the sinuses of Valsalva, from which arise the coronary arteries, and (iii) the sinotubular junction, which is the junction between the superiormost aspect of the aortic root and the ascending aorta.
The ascending thoracic aorta is defined as the portion of the aorta in between the sinotubular junction and the first branch vessel of the aortic arch. The transverse arch is defined as the portion of the aorta, usually horizontal in position, from which the arch vessels arise. The descending thoracic aorta encompasses the bulk of the thoracic aorta and extends from just distal to the last branch vessel (usually the left subclavian artery) to the diaphragmatic hiatus.
Both the sidedness and the branch pattern of the aortic arch may vary. The most common transverse arch is left sided with three branch vessels: the innominate (or right brachiocephalic), the left common carotid, and the left subclavian arteries. The most common variants (Fig. 19.2) of an aortic arch are a common trunk for the innominate and left common carotid arteries (commonly named a “bovine trunk”) and four arch vessels, with either an aberrant right subclavian artery or a separate origin for the left vertebral artery. Approximately 0.05% to 0.1% of people have right aortic arches (1), which may be associated with mirror image branching or an aberrant left subclavian artery (Fig. 19.3). The full scope of aortic arch vascular variants is beyond the scope of this text. Congenital aortic anomalies are described in Chapter 18.
The descending thoracic aorta gives rise to many smaller branches, including the pericardial, mediastinal, bronchial, intercostal, subcostal, esophageal, and superior phrenic arteries (2).
THORACIC AORTIC ANEURYSM
Thoracic aortic aneurysm is defined as an ascending diameter greater than 40 mm, a transverse arch diameter greater than 35 mm, and a descending diameter greater than 30 mm (Fig. 19.4). Normal measurements of the aortic root vary both by age and by gender. More than half of thoracic aortic aneurysms involve the aortic root and the ascending aorta, 10% involve the transverse arch, and 40% involve the descending thoracic aorta (3). It is imperative to recognize the segment (or segments) of the thoracic aorta that are dilated, as the underlying etiology is often location specific. Table 19.1 lists the most common etiologies of thoracic aortic aneurysm by location.
A sinus of Valsalva aneurysm is defined as an aortic cusp diameter greater than >40 mm. 65% to 85% involve the right coronary cusp. Sinus of Valsalva aneurysms are most commonly congenital, arising secondary to incomplete fusion of the aortic media to the aortic annulus, but can also be acquired in the setting of infective endocarditis or as a complication of aortic valve replacement (4). Rupture may have catastrophic sequela such as hemopericardium or myocardial infarction or lead to functional left and/or right ventricular outflow tract obstruction or intracardiac shunting if there is communication with the right atrium (Gerbode defect). Sinus of Valsalva aneurysms can be accurately measured and evaluated with ECG gated CT angiography of the chest or cardiac MR (Fig. 19.5).
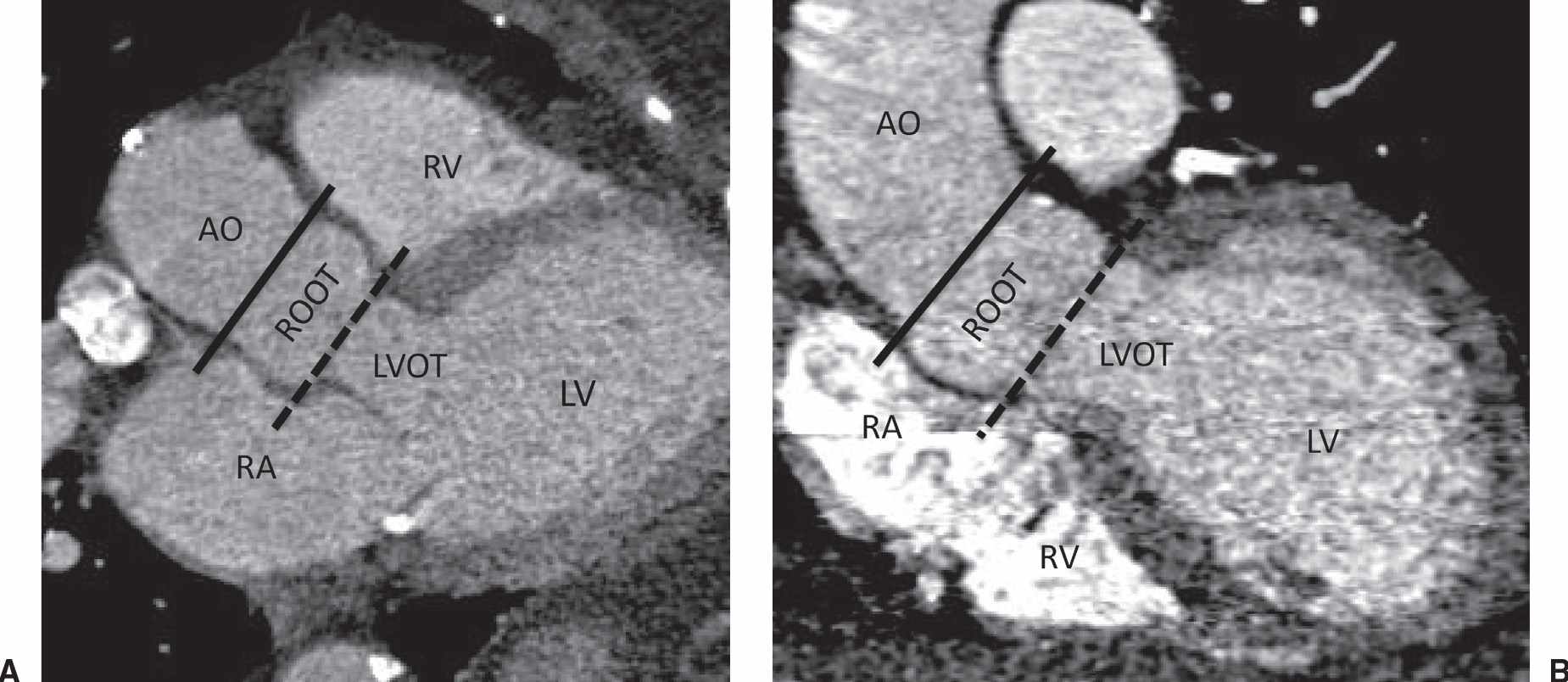
FIG. 19.1 • The aortic root. (A) LVOT multiplanar reformat and (B) coronal aorta multiplanar reformat from a gated CTA of the chest. The aortic root (ROOT) is composed of virtual base ring (dashed line), the sinuses of Valsalva, and the sintotubular junction (solid line). AO, ascending aorta; LVOT, left ventricular outflow tract; LV, left ventricle; RA, right atrium; RV, right ventricle.
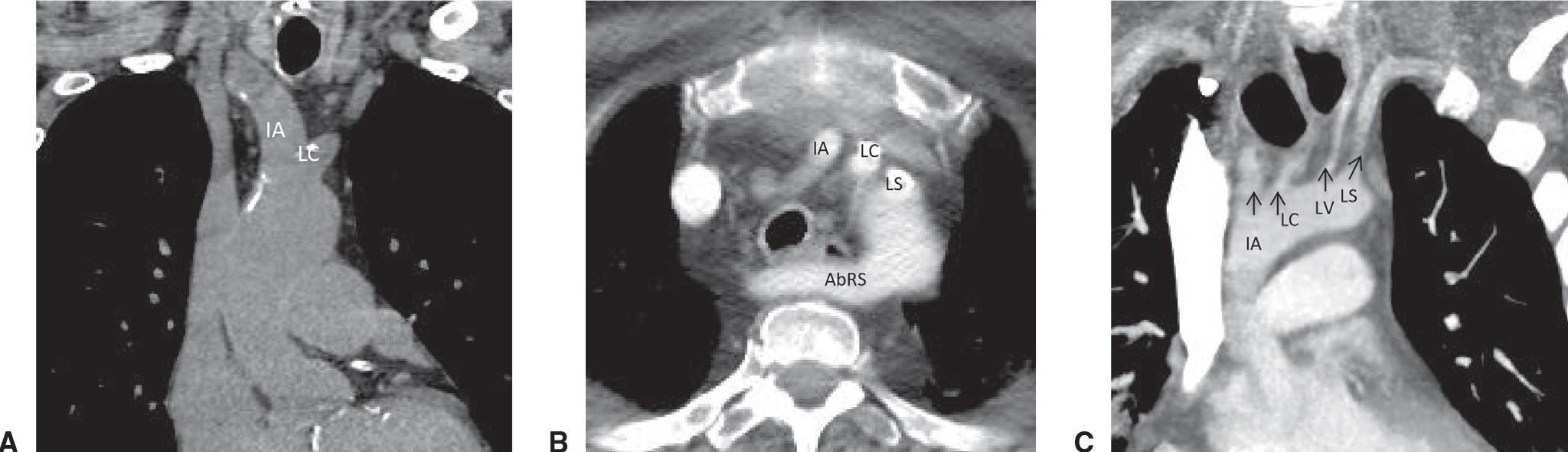
FIG. 19.2 • Common aortic arch anatomic variants. (A) Coronal MPR from a noncontrast chest CT depicts a common trunk for the innominate (IA) and left common carotid (LC) arteries. (B) Axial contrast enhanced CT of the chest demonstrates a left-sided aortic arch with an aberrant right subclavian artery (AbRS). (C) Coronal oblique MIP from a contrast-enhanced chest CT demonstrates a separate origin for the left vertebral artery. AbRS, aberrant right subclavian artery; IA, innominate artery; LC, left common carotid artery; LS, left subclavian artery; LV, left vertebral artery.
The combination of aortic root and ascending aortic dilation is termed annuloaortic ectasia. Causes include hereditary connective tissue disease (e.g., Marfan syndrome or Loews–Dietz syndrome), nonhereditary cystic medial necrosis, infection, and noninfectious inflammation (e.g., Takayasu arteritis) (5). Sole ascending aortic aneurysms can be caused by a bicuspid aortic valve, aortic stenosis, atherosclerosis and hypertension, as well as the etiologies of annuloaortic ectasia.
Atherosclerosis is the most common etiology of descending thoracic aortic aneurysms, and atherosclerotic aortic aneurysms most often occur at the junction of the transverse arch and the descending thoracic aorta. Other causes of descending thoracic aortic aneurysms include infection (i.e., mycotic) (Fig. 19.6), trauma, and inflammatory aortopathies such as those resulting from rheumatoid arthritis and ankylosing spondylitis. Aneurysms can be described as fusiform if there is longitudinal enlargement or saccular if there is focal outpouching (Fig. 19.6).
The location, initial size, rate of annual enlargement, and underlying etiology all factor into if and when the patient should undergo thoracic aortic aneurysm repair. CT imaging features suggesting impending rupture include a crescentic high-density intramural hematoma (see next section), discontinuity of intimal calcifications, and draping of the aorta along the spine (6).
ACUTE AORTIC SYNDROME
Acute aortic syndrome (AAS) is a heterogeneous group of pathologies usually seen in patients with moderate to severe hypertension that present as searing acute migratory chest pain (7). Anterior neck, chest, and throat pain are more commonly associated with AAS involving the ascending aorta, whereas back and abdominal pain is more commonly associated with acute dissection of the descending thoracic aorta. AAS pathologies include penetrating atherosclerotic ulcer, intramural hematoma, and aortic dissection.

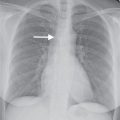
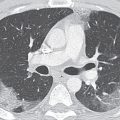
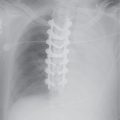
FIG. 19.3 • Right aortic arch with an aberrant left subclavian artery. (A) PA and (B) lateral chest radiographs depict a right aortic arch (solid arrow) and a prominent retrotracheal soft tissue density (dashed arrow) corresponding to a large diverticulum of Kommerell. (C) and (D) Axial contrast enhanced CT of the chest depicts the above findings in more detail. AbLS, aberrant left subclavian; DK, diverticulum of Kommerell.
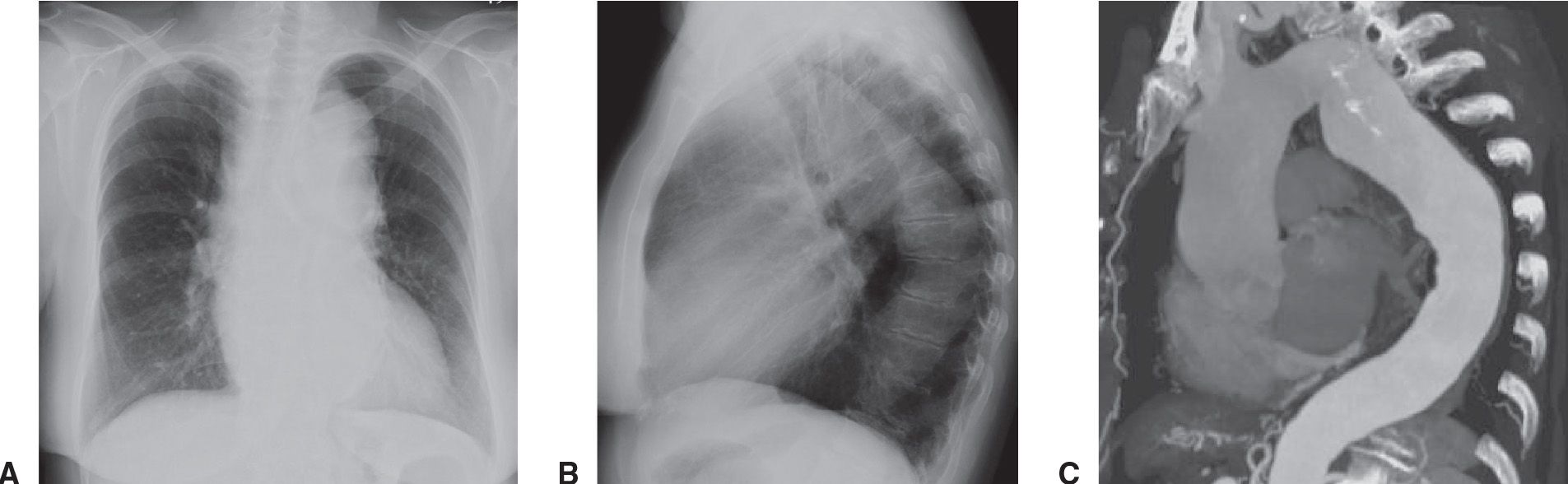
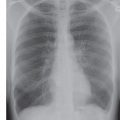
FIG. 19.4 • Ascending and descending thoracic aortic aneurysm. Frontal (A) and lateral (B) chest radiographs demonstrate diffuse tortuosity and enlargement of the thoracic aorta. (C) A double sagittal oblique maximum intensity projection (MIP) image demonstrates a fusiform aneurysm of the entire thoracic aorta.
Table 19.1 THORACIC AORTIC ANEURYSM ETIOLOGIES BY LOCATION
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree