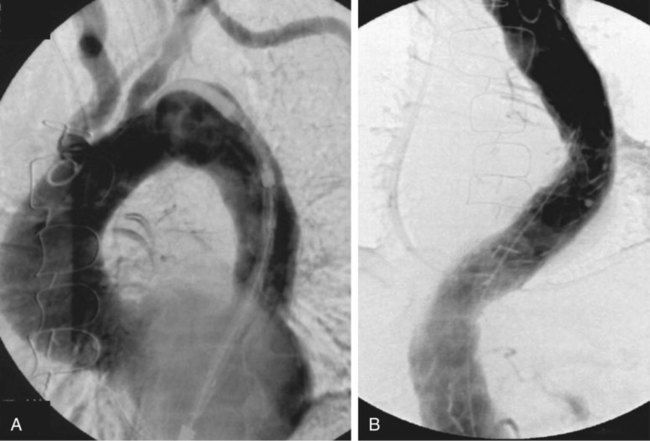
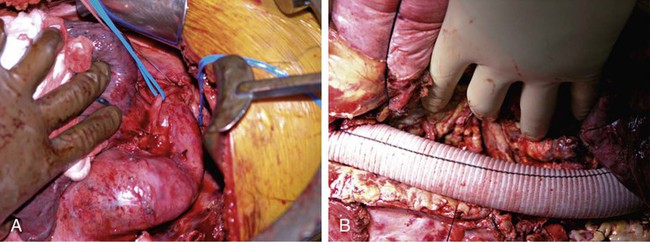
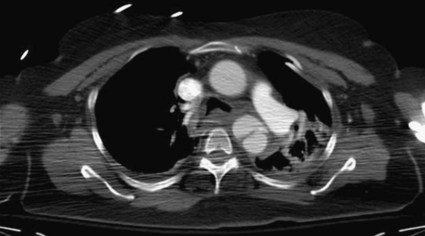
Nina M. Bowens, Edward Y. Woo, Mark A. Farber and Ronald M. Fairman Treatment of descending aortic aneurysms has been the most common application for thoracic endovascular aortic repair (TEVAR) (Fig. 83-1). However, thoracic stent-grafts are preferentially used for repair of traumatic injuries to the aorta, acute type B dissections complicated by malperfusion, rupture, and failure of medical therapy, and virtually all thoracic aortic pathology when anatomically feasible. This is not surprising given that open surgery mandates a thoracotomy, single-lung ventilation, aortic cross-clamping, typically partial cardiopulmonary bypass, and for very proximal thoracic aortic lesions, circulatory arrest (Fig. 83-2). In the United States, three commercially available devices, including the Gore (Flagstaff, Ariz.) TAG and more recently the conformable TAG (CTAG), the Cook Medical (Bloomington, Ind.) TX2, and the Medtronic (Minneapolis, Minn.) Talent and Valiant, have U.S. Food and Drug Administration (FDA) approval and labeling for treatment of thoracic aortic aneurysms (TAAs). The pivotal trials evaluating these devices clearly demonstrated improved 1-year outcomes compared to open surgery (Table 83-1), and the 5-year TEVAR data are now available and continue to indicate freedom from aneurysm-related mortality.1–8 TABLE 83-1 Summary Data of Pivotal Trials There has been widespread acceptance and utilization of endovascular therapy whereby it has become the preferred approach for thoracic aortic transections and complicated type B dissections at most centers of excellence. Furthermore, it is remarkable that even in the absence of FDA approval, physicians have recognized the broader applications of an endovascular approach and embraced off-label use of TEVAR to treat various thoracic aortic pathologies.9–22 Thoracic aortic stent-grafting evolved from abdominal aortic stent-grafting. Initial reports of TEVAR involved using “homemade devices” or abdominal components in an off-label manner. Dedicated thoracic components have been commercially available in the United States since 2005, and device iteration continues to progress beyond the first generation.23–25 The historical indication for operative intervention has been an aneurysm size of 6.5 cm, based on studies demonstrating significantly increased rupture rates.9 However, with the widespread availability of thoracic endografts, this rigorous anatomic selection criteria may be evolving in real-world practice to earlier intervention for smaller aneurysms. Fusiform and saccular aneurysms have been the most commonly treated pathology, but worldwide, thoracic stent-grafts have been increasingly used for aortic dissection (acute and chronic), penetrating ulcers, aortic (airway and gastrointestinal) fistulas, aortic rupture, and blunt traumatic injury, which has recently received FDA approval in the United States.19–26 Management of traumatic injury to the thoracic aorta or great vessels is often complex. Arterial injury is confined to the thoracic aorta in 81% of cases, affects the aortic branch vessels alone in 16%, and involves both the thoracic aorta and great vessels in 3%.27,28 Penetrating injuries involving these structures frequently results in rapid exsanguination and death. Common causes of penetrating thoracic trauma include stab wounds and medium-velocity bullet wounds. The anatomic location and extent of intrathoracic vascular injury secondary to penetrating trauma is difficult to anticipate, owing to variant depth and trajectory of the penetrating object. Blunt thoracic trauma, although less common, may result in injury to the ascending aorta, aortic arch, descending thoracic aorta, or great vessels. Establishing the diagnosis of injury to the thoracic aorta or great vessels usually begins with considering the mechanism of injury. Frequently, especially in the setting of blunt trauma, multisystemic injury and depressed levels of consciousness limit acquisition of historical data. In these circumstances, algorithmic protocols such as the Advanced Trauma Life Support (ATLS) guidelines allow coordinated assessment, diagnostic evaluation, and resuscitation. As in the elective setting, computed tomographic angiography (CTA) has become the diagnostic study of choice, given its widespread availability and rapid image acquisition time (Fig. 83-3).29,30 Relative contraindications to endovascular repair of the thoracic aorta also exist. Long-term durability of TEVAR has not yet been established, so its applicability in the young patient remains controversial. Historically, open repair was used for unstable patients who were unable to undergo preoperative imaging. However, the Vascular Group at Albany established the safety of stent-grafting in traumatic rupture of the thoracic aorta, and practice guidelines developed by the Society for Vascular Surgery continue to support the use of TEVAR for traumatic thoracic aortic injury.31,32 The concern of contrast-induced nephropathy in patients with concomitant thoracic aortic pathology and renal insufficiency can be successfully circumvented by using intravascular ultrasound (IVUS), allowing TEVAR to be performed with minimal use of contrast material. Achieving a proximal seal is frequently more challenging than obtaining a distal seal. This is due to several factors, including angulation of the distal aortic arch and proximity of the great vessels. In most patients, the likelihood of achieving satisfactory proximal and distal seal zones can be determined by carefully evaluating preprocedural CT images, correlating this information with intraprocedural angiographic data, and having a low threshold for performing partial (or complete) over-stenting of the left subclavian artery. Coverage of the left subclavian artery remains a controversial subject because the literature is devoid of randomized prospective data. Most would support coverage of the left subclavian in the emergency setting, with selective revascularization based on vertebral anatomy following TEVAR. However, guidelines in the elective setting remain in evolution.32,33 The Gore TAG was the first FDA-approved device for the treatment of thoracic aneurysms. Design revisions in 2004 (spine removal) simplified its use and significantly decreased late graft-related complications. Five-year results comparing endovascular treatment using the Gore TAG with open surgical repair demonstrated significantly reduced mortality and major adverse events (MAEs) in patents undergoing TEVAR.8 Medtronic completed its pivotal VALOR trial of the Talent thoracic stent-graft, demonstrating TEVAR superiority to open surgical repair.1 Trials evaluating the Valiant, which involves modifications and enhancements to the Talent graft, similarly published favorable early and midterm results for stent use in a wide variety of thoracic aortic pathologies.2,3 The FDA approved the Medtronic Valiant device in 2011. The Cook Medical Zenith TX2 trial also succeeded in demonstrating non-inferiority of TEVAR over open surgical repair.4,5 Moreover, it is important to recognize that following initial FDA approval, these devices undergo further iteration over time as industry responds to physician feedback (Table 83-2). TABLE 83-2 Thoracic Endovascular Graft Device Iterations FDA, U.S. Food and Drug Administration; PTFE, polytetrafluoroethylene. The various grafts have design features that make them unique. Each has a different release mechanism during endograft deployment. The Valiant (Medtronic) offers a proximal self-expanding polished nitinol wire frame for fixation and is available in larger diameters that allow treatment of aortas up to 42 mm. This graft has been modified to include the addition of an eight-peak spring (compared to a five-peak spring in the Talent system) to allow for improved proximal fixation and elimination of the connecting bar for increased flexibility. The proximal bare spring remains constrained following deployment of the stent-graft and is released subsequently. The Zenith (Cook) device uses a completely covered proximal stent and an uncovered stent distally, with barbs both proximally and distally for improved fixation; in the regular graft sizes, it also has a tapered graft. This graft may be used as a one- or two-component system. Two-year data examining use of the TX2 Zenith graft suggest that the two-component system is employed in more complex patients and thus associated with increased perioperative morbidity.5 The recently improved conformable TAG (Gore CTAG) device incorporates a short uncovered stent proximally for better wall apposition and an unflared straight distal configuration. The enhanced device has improved compression resistance and is manufactured in smaller diameters and tapered designs. In addition, a novel trilobed balloon to allow continuous aortic flow during balloon inflation is also available as an accessory. Moreover, the choice of endograft should certainly be tailored to individual patient specifications such as aneurysm anatomy and access availability. Despite the tubular design, thoracic endografting can often be more challenging than abdominal endografting, so it is critical to have the necessary equipment, facilities, and personnel during procedures. Standard wires and catheters are necessary for obtaining access. Stiff wires, such as the Lunderquist (Cook), are important for device tracking. The devices of all manufacturers can be quite stiff and difficult to track through a tortuous aorta or around the aortic arch. All wires must be at least 260 cm for access from the groin. Marker pigtail catheters can be helpful for intraprocedural length measurements. Balloons used for graft effacement are generally device specific. At times, a noncompliant aortic balloon may be necessary (Table 83-3). TABLE 83-3 Suggested Equipment for Endovascular Management of Traumatic Thoracic Aortic Injuries Ideally, these procedures should be performed in an endosuite within a fully functional operating room. Certainly, a mobile system can suffice, but the resolution with a fixed platform is superior. Operating room capabilities are important because most of these procedures require at least one femoral exposure, and retroperitoneal access to the iliac artery is often needed.7,34 Notably, gender analysis of the Talent system revealed that iliac conduits are required significantly more often in women than men.35 In addition to vascular access concerns, emergency situations requiring an open operative procedure can arise, necessitating operating room capabilities. Assembly of an appropriate operative team is paramount for successful aortic repair and patient outcomes. Technicians familiar with the devices, equipment, and procedural steps are helpful, and anesthetic support is critical. Patients often need invasive monitoring for strict hemodynamic control, especially when working around the arch. Moreover, spinal drains may be necessary to decrease intraspinal pressure and help prevent paraplegia. Neurologic support is also indicated and may be in the form of monitoring motor or somatosensory evoked potentials. Studies have clearly shown that monitoring of such potentials can help manage the incidence of paraplegia, at least during open repair.36–39
Thoracic Aortic Stent-Grafting and Management of Traumatic Thoracic Aortic Lesions
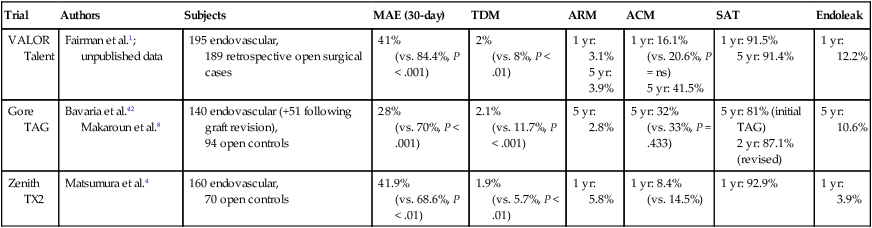
Trial
Authors
Subjects
MAE (30-day)
TDM
ARM
ACM
SAT
Endoleak
VALOR
Talent
Fairman et al.1; unpublished data
195 endovascular,
189 retrospective open surgical cases
41%
(vs. 84.4%, P < .001)
2%
(vs. 8%, P < .01)
1 yr: 3.1%
5 yr: 3.9%
1 yr: 16.1%
(vs. 20.6%, P = ns)
5 yr: 41.5%
1 yr: 91.5%
5 yr: 91.4%
1 yr: 12.2%
Gore TAG
Bavaria et al.42
Makaroun et al.8
140 endovascular (+51 following graft revision),
94 open controls
28%
(vs. 70%, P < .001)
2.1%
(vs. 11.7%, P < .001)
5 yr: 2.8%
5 yr: 32%
(vs. 33%, P = .433)
5 yr: 81% (initial TAG)
2 yr: 87.1% (revised)
5 yr: 10.6%
Zenith TX2
Matsumura et al.4
160 endovascular,
70 open controls
41.9%
(vs. 68.6%, P < .01)
1.9%
(vs. 5.7%, P < .01)
1 yr: 5.8%
1 yr: 8.4%
(vs. 14.5%)
1 yr: 92.9%
1 yr: 3.9%
Indications
Contraindications
Preoperative Evaluation
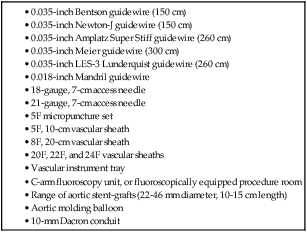
Equipment
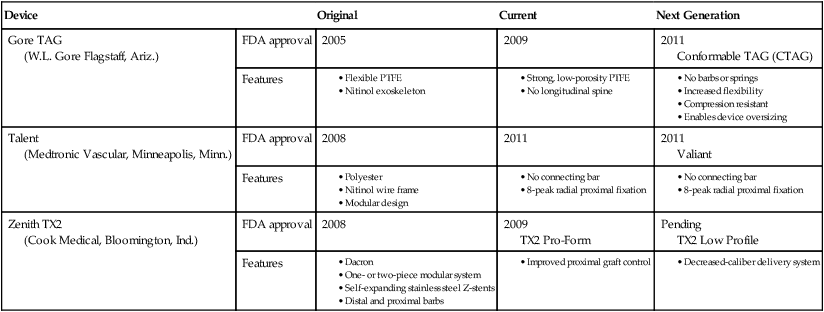
Device
Original
Current
Next Generation
Gore TAG
(W.L. Gore Flagstaff, Ariz.)
FDA approval
2005
2009
2011
Conformable TAG (CTAG)
Features
Talent
(Medtronic Vascular, Minneapolis, Minn.)
FDA approval
2008
2011
2011
Valiant
Features
Zenith TX2
(Cook Medical, Bloomington, Ind.)
FDA approval
2008
2009
TX2 Pro-Form
Pending
TX2 Low Profile
Features
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine