Clinical management of malignancies of the chest
Lung cancer
Lung cancer is the leading cause of cancer deaths in men and women worldwide with a 5-year survival of only around 20% ( ). Cigarette smoking is the single most common risk factor for the development of lung cancer, but air quality, residential radon, and occupational exposures such as asbestos rank among the other major factors ( ; ). In areas with more stringent cigarette use policies, the incidence has been decreasing, particularly in men ( ). However, areas of poorer air quality and increases in women smokers over the last century have resulted in a higher prevalence of lung cancer in women, overtaking breast cancer mortality in the last decade. While tobacco cigarette smoking has been decreasing in developed countries, the legalization of marijuana smoking and vaping may maintain high incidences of lung cancer in the future in those countries ( ; ).
Because early lung cancer is largely asymptomatic, by the time symptoms arise, the tumor will have already become locally advanced or metastatic and 80% of patients are being diagnosed at these stages. This is a major reason for the poor 5-year survival. Indeed, patients with stage I resected lung cancer have a much better 5-year survival of around 80% ( ). With the more generalized use of CT scans, more lung cancers are now being found incidentally and survival following resection of these incidentally found cancers stage-matched to those found due to symptoms is higher, suggesting some differences in biology ( ). Because survival is so strongly linked to stage, there is a strong basis for the development of lung screening programs in people at high risk. The National Lung Screening Trial utilized low-dose CT scans of the chest, in comparison to chest radiograohy, to screen for lung cancer in more than 50,000 participants ( ). There were 247 deaths from lung cancer per 100,000 person-years in the low-dose CT group and 309 deaths per 100,000 person-years in the chest X-ray group, representing a relative reduction in mortality from lung cancer with low-dose CT screening of 20% ( ).
With the identification of a lung nodule suspicious for lung cancer, a tissue diagnosis needs to be established. CT scans of the chest, abdomen, and pelvis allow for an initial staging and also for selecting a method for biopsy. Widely metastatic disease may allow for tissue diagnosis from thoracentesis of a malignant pleural effusion, bedside fine needle aspiration of supraclavicular lymph nodes, or ultrasound-guided liver biopsy, as examples. If distant metastatic disease is not identified, tissue sampling of mediastinal or hilar lymph nodes can be achieved by endobronchial ultrasound-guided fine-needle aspiration ( ). If the tumor appears confined to the lung, CT-guided lung biopsy or radial endobronchial ultrasound-guided biopsy, if close to an airway, can be used. The diagnosis of lung cancer can be made on cytologic or histopathologic samples, but histopathologic samples are preferable to a cytologic specimen as it provides extraarchitectural information and usually yields more tissue for immunohistochemical and genetic analysis of the tumor ( ). As endobronchial ultrasound-guided aspiration can only generate cytological specimens, advances in immunocytochemistry are improving the diagnostic yield of these specimens ( ). Among lung cancers, the major first distinction is between small-cell (SCLC) and non–small-cell (NSCLC) histologies due to the marked difference in the clinical course. SCLC is a high-grade neuroendocrine tumor that occurs almost exclusively in smokers and is characterized by rapid growth and early development of metastases ( ). These tumors are also frequently associated with paraneoplastic syndromes such as syndrome of inappropriate antidiuretic hormone secretion, Cushing syndrome, Lambert–Eaton syndrome, encephalomyelitis, and sensory neuropathy syndromes ( ). The NSCLC group includes adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Because these tumors generally follow a similar clinical course, they are grouped together despite different histologies.
The clinical stage needs to be established next. Lung cancer follows the primary tumor, regional lymph node, distant metastasis (TNM) standard of staging according to the eighth edition of TNM (AJCC-UICC) ( ). Unlike many other cancers, the prevalence of lung cancer and the presence of an international database of patients and their outcomes means that stage classifications are data-driven. The International Association for the Study of Lung Cancer used 94,708 lung cancer patients and their outcomes for stage groupings ( ).
Clinical staging is largely imaging-based. CT scans are usually already available once a tissue diagnosis is made and are helpful for both T-staging of the primary, based on size and location, and M-staging for distant disease. In most cases, N-staging can also be identified by bulky enlarged mediastinal or hilar lymph nodes, but microscopic involvement greatly affects staging, and invasive mediastinal staging of lymph nodes by cervical mediastinoscopy or endobronchial ultrasound is needed for confirmation ( ). In most centers, an 18 F-fluorodeoxyglucose (FDG) PET/CT scan is routinely performed as it has been shown to be more accurate in the detection of mediastinal nodal disease and can detect occult metastatic disease not identified by CT ( ). Some argue therefore that routine invasive mediastinal staging precludes the need for PET/CT; however, studies have shown the impact of PET/CT in changing clinical care and current guidelines for the workup of lung cancer include PET/CT ( ; ; ).
Because brain metastases may be hyper- or hypo-metabolic, FDG-PET is less useful for the identification of brain metastases. Therefore, gadolinium-enhanced MRI of the brain is preferred in stage III and IV patients ( ). While TNM staging exists and remains recommended for the staging of SCLC, due to the cancer’s rapid dissemination potential, a treatment-based staging system is often utilized clinically. In this system, SCLC is separated into “limited stage” disease which means that the cancer is on one side of the chest and can be treated with a single radiation field, and “extensive stage” which means the cancer has spread beyond limited stage ( ). This system helps separate patients treated with chemoradiotherapy from those treated with chemotherapy but does not separate out the rare early stage patients from the much more common locally advanced disease.
SCLC tumors are very responsive to chemotherapy. The very rare patient without nodal or distant metastases can undergo surgery followed by adjuvant chemotherapy and consideration of prophylactic cranial irradiation (PCI). Because this population is so rare, there are few randomized controlled trials comparing surgical to nonsurgical approaches and comparing outcomes with and without PCI ( ). For the more common situations of limited-stage SCLC, chemoradiotherapy is the established first line of treatment ( ). PCI has been shown to decrease the risk of symptomatic brain metastases in this population and increase overall survival ( ). In patients with extensive stage disease, chemotherapy alone using a platinum agent with etoposide is the standard of care. Radiation is reserved for palliation and PCI is not obviously beneficial in this setting ( ). For NSCLC patients, surgery is the mainstay of treatment in stage I and II disease. Lobectomy, resection of a single lobe of the lung with formal division of the pulmonary artery, bronchus, and pulmonary vein of that lobe, is the standard operation ( ). However, with the advent of CT and the development of lung cancer screening programs, smaller tumors are being detected more frequently. As many have favorable histology, sublobar resection may yet be adequate and preserve lung parenchyma. A review of SEER and NCDB database data demonstrated a survival benefit with lobectomy ( ; ; ; ; ). Adjuvant or neoadjuvant chemotherapy is offered to stage II disease and in selected stage IB patients with high-risk features ( ). For stage III disease, the mainstay of therapy is concurrent chemoradiation ( ). Patients with single nodal station, microscopically positive disease may benefit from surgery afterward, but patient selection is critical ( ). Stage IV disease is treated with systemic therapy and targeted palliative treatments.
Around 20 years ago, the molecular pathways which drove NSCLC malignancy began to be elucidated ( ). Certain recurrently mutated genes in molecular signaling pathways were identified such as in the epidermal growth factor receptor (EGFR) gene, the anaplastic lymphoma kinase (ALK) gene, or c-ROS oncogene one gene, among many others ( ). These mutations are known as “driver mutations” which indicate that they are important in converting a noncancerous cell to a malignant one and that the resulting malignant cell relies on this mutated cell signaling for continued survival. Consequently, these mutations are desirable therapeutic targets, and therapies that aim to disrupt these pathways are known as “targeted therapies.” While tumors will ultimately develop escape mutations that render treatment ineffective, in general, tumors with a targetable mutation have better prognoses ( ).
Targeted therapies now exist for a variety of NSCLC mutations. EGFR mutation is observed in ∼15% of lung adenocarcinomas in the US but is markedly higher in the Asian population ( ). In advanced NSCLC with EGFR mutations, EGFR tyrosine kinase inhibitors (TKI) targeting these mutations are more effective than conventional chemotherapy and are considered the front-line treatment ( ; ). Multiple generations of EGFR TKIs have now been developed ( ). Other mutations include ALK rearrangements which are present in ∼4% of NSCLC adenocarcinomas and can be targeted by ALK TKIs ( ). In 1–2% of patients, ROS1 rearrangements can act as a driver oncogene when translocated with other genes such as the CD74 gene. ROS1/MET inhibitor crizotinib and ROS1/TRK inhibitor entrectinib are used in the front-line setting for these mutations ( ). MET amplifications independent of ROS1 can also be treated with crizotinib.
As targeted therapies evolve, testing for these mutations must evolve along with them ( ; ).
Novel methods of detecting lung cancer are now actively being developed. One promising method is the “liquid biopsy” which obviates the need for tumor biopsies ( ). Tumor cells shed compounds into the blood which can then be detected, demonstrating the existence of the tumor. Currently, circulating tumor DNA (ctDNA), that is, DNA from dying tumor cells shed into the blood, and circulating tumor cells, that is, tumor cells shed into the blood are the most promising candidates ( ). Because NSCLC mutations are so well defined, ctDNA can be identified on the basis of those mutations ( ). Tests for ctDNA containing EGFR mutations have been developed. There is considerable expectation that these tests will allow for tumor detection, evolution tracking, and recurrence in the future.
Though many driver mutations have been identified in NSCLC, many tumors will still lack known mutations. In this setting, conventional chemotherapy has remained the sole choice of therapy until recently. Immune checkpoint inhibitors are a recent class of drug which targets the physiologic brake of immune activation. Common examples include programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) inhibitors ( ). When PD-L1 interacts with PD-1, it inhibits T effector cells and promotes the conversion of T effector cells to T regulatory cells. Therefore, by expressing PD-L1, tumors can escape host immune surveillance. PD-1 and PD-L1 inhibitors, therefore, act to break this mechanism of immune escape. In advanced NSCLC, PD-L1 expression by the tumor is tested by immunohistochemistry. Pembrolizumab is currently approved for the front-line treatment of patients with EGFR/ALK wild-type NSCLC with PD-L1 expression >50%. In the KEYNOTE-024 trial, where these patients were randomized to single-agent pembrolizumab versus platinum chemotherapy, both progression-free survival (10.3 vs. 6 months) and medial overall survival (26.3 vs. 13.4 months) were markedly better with immunotherapy ( ). In patients with low PD-L1 expression, PD-1 or PD-L1 blockade has still demonstrated benefit over chemotherapy. The CheckMate-227 trial showed that nivolumab and ipilimumab allowed longer overall survival compared to chemotherapy (17.1 vs. 13.9 months) ( ). Multiple trials continue to define how immunotherapy will best be used to treat NSCLC. Indeed, trials attempting neoadjuvant immunotherapy may redefine how resectable NSCLC will be treated in the future ( ).
In summary, lung cancer is currently the most common cause of cancer mortality in men and women around the world. NSCLC is treated based on the stage at presentation, with early disease treated primarily with surgery and locally advanced disease treated with chemoradiation. Advanced disease is treated palliatively with systemic therapy and palliative treatments. Immunotherapy has led to a paradigm shift in the treatment of NSCLC and neoadjuvant in the future. In contrast, SCLC is much more aggressive than NSCLC and systemic chemotherapy is the mainstay of treatment and radiation added for limited-stage disease.
Pulmonary metastases
Being the first microvascular bed encountered following the return of blood to the heart, the lungs are a common location for metastatic deposits ( ). In most patients, this signifies widespread dissemination and, therefore, advanced disease. However, in certain situations, these lung metastases are isolated. Therefore, resection of these isolated metastases may potentially lead to prolonged survival or even cure ( ). Because of the myriad malignancies which can metastasize to the lungs and their variable individual tumor biology, studies are difficult to design, and whether metastasectomy actually impacts patient survival remains unknown. Indeed, the only published pulmonary metastasectomy randomized clinical trial was “PulMiCC” which randomly assigned 65 patients with colorectal cancer metastases to the lung and could not recruit the complete cohort ( ). There are, however, clinical case reports and case series which report prolonged survival and long-term relapse-free survival ( ).
The decision to proceed with pulmonary metastasectomy therefore currently largely depends on clinical judgment. Ideally, a multidisciplinary team including a medical oncologist, radiation oncologist, and thoracic surgeon should define the indication and optimize the timing of pulmonary metastasectomy. General criteria for considering resection are as follows ( ):
- 1.
The pulmonary metastases are isolated and appear to be completely resectable on imaging.
- 2.
Metastasectomy is technically feasible and the remaining pulmonary tissue is adequate for the patient’s pulmonary function.
- 3.
The primary tumor is controlled or controllable.
- 4.
The lung is the only site of metastatic disease or extrapulmonary metastatic disease is controllable.
Certain additional considerations can influence prognosis. One such is that of disease-free survival. Outcomes are better when there has been a longer disease-free interval between primary tumor control and presentation of pulmonary metastases, that is, a patient who presents with pulmonary metastases 3 months after primary resection will usually fare poorer than one who presents 3 years later. Data from the International Registry of Lung Metastases demonstrate a higher 5-year survival for those with a disease-free interval greater than 36 months than those with less than 12 months (45 vs. 33 months) ( ). Therefore, unlike with primary lung cancers, often it is better to delay resection of lung metastases for a few months to ensure that additional lesions do not arise in the meantime. Outcomes are also better with fewer metastases. Though “complete” resection is the aim and therefore numerous metastases can be resected if they are all removed, generally, three or fewer metastases are ideal. The International Registry shows that single metastasectomy has better 5-year survival than the resection of more than three metastases (43% vs. 27%) ( ). The biology of the primary cancer can also influence outcomes. In general, sarcomas metastasize via the vascular route and metastases limit themselves to the lung parenchyma. However, metastases from other common cancers such as colon and breast have the potential to then metastasize to locoregional lymph nodes, limiting resectability ( ).
Given that metastases are rarely symptomatic, detection, and preoperative evaluation rely on imaging. CT is the key modality and allows for detection and operative planning. However, because complete surgical resection is vital for benefit, FDG-PET has become an important modality to rule out occult distant disease, assuming the primary is FDG-avid ( ). Along those same lines, if there is a propensity of the primary tumor to metastasize to the brain, brain imaging with MRI should be performed.
In many cases, surgical resection of all pulmonary metastases is not feasible due to the amount of lung tissue required to be resected. In these situations, stereotactic body radiotherapy (SBRT) can also be used to treat pulmonary metastases and appears to be effective and well-tolerated. In a phase II randomized trial between SBRT and palliative standard-of-care treatment, there was a survival benefit with SBRT (41 vs. 28 months) ( ). Moreover, with radiation treatment, there is a theoretical additional benefit where radiation-induced tumor death may stimulate the host immune system to generate a tumor-specific response known as the “abscopal effect” ( ). However, this effect remains theoretical and the major downside to SBRT compared to surgery is the lack of confirmed complete eradication of the metastasis and may affect the long-term durability of control. These are challenging questions to address and a systematic review of the literature has not been able to demonstrate a superiority of either approach ( ). Consequently, both surgery and SBRT can be used to treat pulmonary metastases, and approaches that combine SBRT for lesions requiring larger resections with surgery for peripheral lesions currently remain the ideal strategy.
In summary, metastatic disease to the lung is common for a variety of cancers, including sarcoma, colorectal cancer, and breast cancer, among many others. Careful selection of patients for pulmonary metastasectomy may result in increased disease-free survival but has yet to be demonstrated in randomized controlled trials. A tailored strategy for each patient devised through multidisciplinary meetings is the current paradigm.
PET/MRI of malignancies of the chest
Technical considerations and protocols
Chest PET/MRI protocols, besides the need to produce reliable attenuation correction (AC) maps and adequate signal-to-noise ratio MR images, have to also take into consideration the unique challenges imposed by breathing and cardiac pulsation.
With the recent introduction of short TE imaging, including ultrashort echo-time (UTE) and zero echo-time (ZTE) this could be addressed by providing continuous attenuation coefficients instead of one value per segmented tissue, which can significantly underestimate the radiopharmaceutical concentration ( ). Short echo-time sequences also can not only improve the AC but do offer potentially increased lung lesions detection rates, better resolution as well as improved visualization of the lung parenchyma.
PET/MRI has become widely accepted for various cancer types, however, there is a limitation in the performance of the MRI portion of PET/MRI for the detection of pulmonary nodules and malignancies. Conventional MRI of the lung can be performed either with breath-holding, which limits the spatial resolution, or with free-breathing, which, if not corrected for, results in reduced image quality due to respiratory and cardiac motion ( ). To overcome motion artifacts, navigated options have been introduced, though at the cost of longer acquisition time. Moreover, navigated schemes are prone to failure in patients with irregular breathing rhythm and or irregular heart rate ( ). While some of those issues might be overcome in the future with AI techniques, those are currently in the evaluation phase and not widely applicable or available ( ; ; ; ).
Currently used lung MRI techniques include T1-weighted 3D gradient-echo (GRE) sequences, for example, volume-interpolated breath-hold examination or liver accelerated volume acquisition (LAVA), T2-weighted half-Fourier single-shot turbo spin-echo (HASTE), and diffusion-weighted imaging (DWI) ( ; ). T1-weighted 3D GRE sequences are relatively motion robust, able to depict even subcentimeter pulmonary nodules, and have proven to be ideal for identifying lymph nodes ( ; ; ; ). Gated and partly motion-corrected T2w sequences are superior in the detection of tumor infiltration, however, a combination of T1w 3D GRE and T2w HASTE can also increase lung nodule detection and provide synergistic effects ( ; ). Since both types of sequences need to be acquired, this can result in disadvantages in acquisition time.
Recently introduced respiration-gated T2-weighted-PROPELLER (Periodically Rotated Overlapping Parallel Lines with Enhanced Reconstruction) sequences have even higher detectability for lung nodules compared to T1-weighted MRI sequences, with the disadvantage of additional scan time investment ( ). The potential added value of T1w 3D postcontrast GRE sequence to PET/MRI for the detection of pulmonary nodules is still under debate ( ; ). Promising MRI techniques, such as short echo time imaging UTE and ZTE for this aspect, will be discussed later.
Apart from the MRI sequence choice, further challenges of imaging the lung parenchyma are caused by the large field-of-view and required high resolution for diagnostic interpretation, demanding long acquisition times. A possible solution includes parallel imaging which can decrease overall scan time, at the expense of decreasing the signal-to-noise ratio (SNR). Although parallel imaging has been optimized for cartesian sequences, the integration in noncartesian protocols like UTE is still lacking implementation, due to the demanding image reconstruction. However, noncartesian parallel imaging could significantly shorten the scan time ( ).
Routine PET acquisition contains only a few coincident events, resulting in inherently noisy imaging quality. In part due to advances in processing hardware, the past decade has seen a surge in research focused on machine learning and artificial intelligence. A recent study evaluated the use of convolutional neural networks (CNN) for improving the noise properties of PET images reconstructed from low-count data in lung cancer patients ( ). The results of the study indicate that in terms of image quality, the CNN-denoising offered several improvements over the original images, including better noise properties and improved measurement reproducibility of pixel values. In general, these images were also consistently ranked higher than original low-count images in the clinical evaluation, leading to a superior lesion detectability rate. Another study by Li et al. demonstrated the feasibility of a motion correction method for respiratory-gated PET images using a deep learning based image registration framework ( ). The proposed method using simulation and clinical data showed its ability to reduce motion artifacts while utilizing all gated PET data.
Current clinical PET/MRI aspects
Lung cancer is one of the leading causes of cancer-related mortality worldwide and accurate staging is crucial for appropriate management and prognosis ( ). PET/CT has proven indispensable in the staging, therapy, follow-up, and prognostication of non–small cell lung cancer (NSCLC) and has been widely adopted in clinical practice, providing also useful data for the characterization of morphologically indeterminate pulmonary nodules.
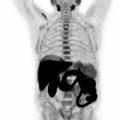
Lately, fully integrated PET/MRI has transitioned from a predominantly research focus into clinical routine, holding great promise as an innovative hybrid imaging modality in several cancer forms, especially of the abdomen and pelvis, given the high soft tissue contrast of MRI ( ; , , ; ; ; ).
The main advantages of the technique include multiparametric functional and morphological as well as metabolic information, provided by the combination of radioactive tracers with different MRI sequences. Moreover, the absence of radiation exposure in MR imaging is a major benefit, especially when repeated studies are performed in the management of oncologic patients.
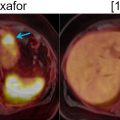
While PET/MR imaging has been shown to outperform PET/CT in the oncologic workup of the abdomen and pelvis in many solid organ malignancies, the evaluation of pulmonary nodules is limited ( ; ; ). The main reasons for the limited performance are the low proton densities of the lung parenchyma, fast transverse magnetization decay as well as cardiac and respiratory motion artifacts ( ; ; ). The application of integrated PET/CT, combining anatomic and metabolic imaging information has demonstrated excellent performance in the detection and classification especially of smaller pulmonary nodules based on the availability of the CT component ( ). Kim et al. confirmed the high accuracy of FDG-PET/CT in the classification of solid pulmonary nodules as benign or malignant with sensitivity and specificity of 97% and 85% compared to 93% and 31% for CT alone without the metabolic information of PET ( ). However, in PET/CT, solid pulmonary nodules smaller than 8–10 mm frequently appear to be false-negative or are not confidently detectable on the PET component only and in this case can be evaluated on the corresponding CT part only ( ).
Compared to PET/CT, the reported sensitivity for the detection of lung nodules is lower with the usage of PET/MRI, ranging between 30% and 83% ( ; ; ; ; ), which holds particularly true for smaller pulmonary nodules. While the lung nodule detection rate of PET/MRI is comparable to that of PET/CT for FDG avid nodules larger than 10 mm, the PET/MRI detection rate significantly decreases for non-FDG avid nodules smaller than 5 mm, which are admitting of unknown clinical significance in oncologic patients ( ; ). Another recent study, that assessed the feasibility of PET/MRI for lung cancer staging, also demonstrated diagnostic accuracy similar to that of PET/CT in patients with NSCLC, however showing even better sensitivity than PET/CT in the setting of distant metastatic disease, including brain and liver metastases ( ). Importantly, in NSCLC, it has been shown that treatment of early brain metastases, while still asymptomatic, is associated with better control of neurological manifestations and longer survival ( ).
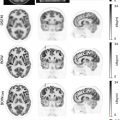
Of particular importance is the optimal staging in a presurgical stage to perform surgery or multimodality treatment only in those patients who might benefit from such therapy and avoid potentially futile surgery and related morbidity as well as unnecessary costs. Messerli et al. found that PET/MRI provides comparable diagnostic value for the determination of tumor resectability in patients with NSCLC compared to PET/CT, using a dedicated pulmonary MRI protocol including a T1-weighted sequence water-only (i.e., LAVA-Flex in breath-hold) and the respiration gated T2-weighted (i.e., PROPELLER) ( ). Thereby, the introduction of PET/MRI, specifically the usage of contrast-enhanced sequences, may also eventually overcome the drawback of PET/CT in the assessment of mediastinal invasion, pleural infiltration, infiltration of the diaphragm or adjacent lobe ( ; ).
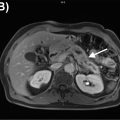
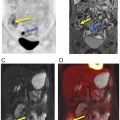
Similar results were reported by Fraioli et al., using a combination of surgical information and an enhanced reference standard derived from CT, PET/CT, and histopathology to assess the performance of PET/MRI in the evaluation of lung cancer resectability ( ). For this purpose, PET/MRI demonstrated high specificity and sensitivity of 92.3% and 97.3%, respectively. Comparing PET/MRI with the reference standard for T-staging, current research showed that the technique slightly overestimates T1 and T2 stages but appeared to underestimate higher stages. Good agreement between the reference standard and PET/MRI was, however, found regarding N staging and M0 staging, whereas M1 staging showed minor discrepancies ( ). In the aforementioned study, the authors applied a practical standard MRI protocol, including large field-of-view T1-weighted and T2-weighted sequences, as well as DWI and whole-body gadolinium contrast-enhanced T1-weighted MRI scan combined after PET acquisition, however, optimal diagnostic protocols are still a matter of debate Figs. 7.1 and 7.2 . Particularly, newly developed, specific dedicated lung protocols aim to further enhance the diagnostic value of the MRI component ( ). It is important to notice that PET/MRI offers not only possible medical/staging advantages. There are also distinct logistical advantages to consider. Patients being referred for the staging of NSCLC (either for PET/CT or PET/MRI) will always already have undergone a chest CT. What is needed from the hybrid imaging procedure now is the overall staging, including a brain MRI up from stage IB. The brain MRI can be obviously integrated into the PET/MRI, without the need for a second appointment, no second injection of contrast material, and therefore providing increased efficiency and potential cost savings. Thus, the patient and the referring physician can be guided effectively and efficiently through the diagnostic algorithm. Most importantly, the increased efficiency may also result in improved survival since it has been shown that repeat PET/CT in patients with NSCLC waiting for treatment can lead to upstaging in up to 13%–18% of patients.