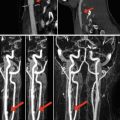
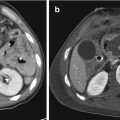
Fig. 13.1
(a–c) CT-angiography showing intimal tear at the aortic istmus (arrows); (d) DSA confirms the presence of an intimal tear (arrow); (e, f) placement of the stent graft; (g) follow-up CTA showing the correct placement of the stent graft with disappearance of intimal tear
13.2.3 Indications and Guidelines
According to the most recent consensus of the Society for Vascular Surgery (SVS) the use of TEVAR is supposed to follow some guidelines that the committee suggested on the basis of a systematic review and meta-analysis of the literature including 7768 patients from 139 studies [29]. As it was said before, only observational studies, case series and unsystematic observations or expert opinion are available to date; thus, using the GRADE system, all of the following indications should be regarded as Grade 2, Level C statements:
- 1.
Timing of TEVAR in a stable patient
The committee suggests urgent (24 h) repair in the absence of other serious concomitant non-aortic injuries, or repair immediately after other injuries have been treated, but at the latest prior to hospital discharge [30, 31]. This is consistent with the available evidence in which mortality was 46% in those managed nonoperatively [32].
- 2.
Management of “minimal aortic injury”
Based on imaging, BTAI can be classified in four grades of severity:
type I: intimal tear
type II: intramural hematoma
type III: pseudoaneurysm (presence of a thin layer of adventitia that covers a transverse tear in the intima and media preventing complete rupture)
type IV: rupture
The committee suggests expectant management with serial imaging for type I injuries, while types II to IV should be repaired. This is consistent with the evidence that most type I injuries can be managed with medical therapy (anti-impulse control). Decision to intervene and its timing should be guided by progression of the initial radiographic abnormality and/or symptoms [33].
- 3.
Choice of repair in the young: TEVAR vs. open
Age should not be a factor influencing the choice of the type of repair. The risks of death and spinal cord ischemia are significantly lower in all age groups after endovascular repair compared with open surgery, and these advantages overcome the concerns of potential late complications [34].
- 4.
Management of left subclavian artery
LSA is generally covered in 30% [32]. The committee suggests selective revascularization (either before or after TEVAR) depending on the status of the vertebral anatomy, especially whether the right vertebral artery is atretic or hypoplastic with or without an intact Circle of Willis.
- 5.
Systemic heparinization
The committee suggested routine heparinization, but at a lower dose than in elective TEVAR.
- 6.
Spinal drainage
Given the low risk of spinal cord ischemia during TEVAR for BTAI treatment (3%) [32], the proximal location of the injury involving limited coverage of the thoracic aorta, and the risk of epidural hematoma in a coagulopathic patient, the committee suggests that spinal drainage is not routinely indicated, and it should only be placed for symptoms of spinal cord ischemia.
- 7.
Choice of anesthesia: general vs. regional vs. local
The committee endorses general anesthesia because of unreliable cooperation of an agitated trauma patient and the presence of concomitant injuries that may require additional surgery.
- 8.
Femoral access technique
The committee suggests open femoral exposure
- 9.
Optimal follow-up strategy
In the absence of any abnormalities on imaging (i.e., stable endograft position, no endoleak) in the first 12–36 months, some have suggested decreasing the frequency to 2–5 years, while others have expressed that, lacking any evidence to the contrary, follow-up for traumatic thoracic aortic injuries should be no different than those treated with TEVAR for other pathologies. There was, however, some consensus suggesting that a combination of a multi-view chest X-ray and a magnetic resonance angiography (MRA) may be preferable over conventional contrast computed tomographic angiography (CTA) for long-term imaging, with due consideration of the metallic composition of the endograft.
13.2.4 Challenges and Limits
Despite TEVAR is associated with a lower risk of complications compared to open repair, there remains a number of controversial issues:
- 1.
Young age of affected population:
As mentioned before, BTAI tends to affect younger populations, in contrast to the aneurysm population. It is not uncommon that adolescent or pediatric patients may present with this injury [29].
This carries some challenges:
Poor conformability of grafts to the aortic arch:
Young patients present not only a smaller aortic diameter, but also a shorter radius of aortic curvature compared to elderly patients. As individuals age, not only does the aorta enlarge, but the radius of curvature also increases [29]. This is potentially problematic for devices that are designed to mimic the stiffness of older patients with thoracic aneurysms. As such, their conformability is not ideal along the lesser curvature of the arch, potentially resulting in proximal endoleaks.
Furthermore, when coupled with the increased aortic impulse in young patients, a lack of proximal conformability can also cause an aortic pseudocoarctation from compression of the device.
Both device collapse and endoleaks are not always predictable and avoidable occurrences. They can, however, be treated when they occur.
Uncertain natural history of the repair:
Given the morphologic changes of the aorta that come with age, the possibility of stent-graft migration as the aorta enlarges must be considered [29].
- 2.
Stent sizing:
Severe hypovolemia can lead to aortic contraction with underestimation of the aortic diameter ranging from 5 to 40% [35]. This can result in potential problems because undersized devices are associated with type I endoleak, persistent lesion perfusion, and bleeding. Oversizing the device would help minimize this risk but excessive oversizing can also lead to major complications as the aforementioned device compression [20].
- 3.
Manipulation of an endograft in the vicinity of the ascending aorta:
It is not only technically difficult but also carries a risk of stroke complications [29].
13.3 Interventional Radiology of Thoracic Wall Arteries
13.3.1 Introduction
Chest wall trauma shows a significant morbidity and mortality rate, with reported mortality ranging from 5 to 25% [3, 4].
Pape et al. [36] developed a scoring system for guiding initial clinical decision-making in the blunt chest trauma patient with multiple associated injuries. However, there are no evidence-based guidelines for management in the blunt chest wall trauma patients without associated injuries.
13.3.2 Clinical Considerations
The chest wall is a typical site of injury in patients with thoracic trauma. Rib fractures, sternum fractures, and flail chest are quite common findings, especially in case of blunt chest trauma. Dislocated fractures can induce damages to the pleuro-pulmonary structures and vessels, with formation of hemothorax, pneumothorax, pulmonary hematoma, pulmonary contusion, and hemomediastinum.
Penetrating injuries of the chest wall are lesser associated with bone fractures, but have similar risk to damage the pleuro-pulmonary structures and vessels.
Bone fractures generally undergo to nonoperative management. An exception is flail chest, for which recent studies suggest major benefits from surgical stabilization [37].
Vascular injury is a quite rare finding in chest wall trauma but its prompt recognition and management is fundamental for patient’s life. Both blunt and penetrating traumas are recognized to be causes of vascular damages of the chest wall arteries.
Thoracotomy was the only useful treatment in these patients until 1977, when Barbaric et al. first reported the effectiveness of TAE for post-traumatic intercostal arterial hemorrhage [38].
13.3.3 Anatomy
The chest wall is the boundary of the thoracic cavity. Arterial supply originates from the intercostal arteries and the internal thoracic arteries [39]. Knowledge of the anatomy is fundamental in case of EVTs because of the high number of collateral vessels that may cause reperfusion and delayed failure of TAE.
The intercostal arteries (11 for each side) are divided into two branches, anterior and posterior. They lie in the intercostal space near the lower margin of the rib, between the intercostal vein (above) and the intercostal nerve (below).
Posterior branches originate directly from thoracic aorta (except for the first and second branch, which arises from the supreme intercostal artery-collateral of the costo-cervical trunk). They give a dorsal branch which feeds the spinal cord (radicular and spinal arteries).
From the first to the sixth, the anterior branches originate from the two internal thoracic arteries, which give rise to the subclavian arteries; from the sixth space the internal thoracic artery divides into the superior epigastric artery and musculophrenic artery. The latter goes downward and laterally and gives out the remaining anterior intercostal branches.
13.3.4 Management
Arterial injuries of the thoracic wall should be suspected in every case of trauma patient with:
Abundant hemothorax/hemomediastinum
Markedly displaced rib fractures
Even if a wide consensus is missing, many authors [40, 41] suggest the following management for these patients.
Patients with chest trauma and evidence of a pneumothorax or hemothorax have to undergo tube thoracostomy. Hemodynamically unstable patients and patients with >200 mL/h of blood loss from the thoracostomy tube should require emergent thoracotomy. When emergent thoracotomy is not indicated, CECT need to be performed. CECT may help to distinguish blood loss from an arterial source versus blood loss from a pulmonary lesion (bleeding from low-pressure pulmonary circulation), which is usually self-limited and in any case cannot be resolved with EVI. If CECT reveals arterial extravasation of contrast medium, angiography with TAE is indicated.
Prompt recognition is important because of the high blood flow in these arteries (150 mL/min for thoracic internal artery), which can be potentially life-threatening in few minutes [42]. Although a lesion of these arteries can achieve temporary hemostasis due to arterial spasm and hypotension, rebleeding may occur after the patient is resuscitated [43].
Active extravasation of contrast medium can be easily detected with CECT, which accurately shows the anatomic location of the bleeding and indicates the probable vascular origin. CECT, therefore, can be used as a guide for angiographic or surgical procedures [44].
CECT can show two types of alterations:
- 1.
Active bleeding: extravasation of contrast medium increasing in the time
- 2.
Pseudoaneurysm: disruption of one or more layers of the arterial wall forming a perfused sac with contrast medium that washes away with bloodstream
Both these lesions require an invasive treatment because pseudoaneurysms are by definition instable lesions and may develop in a real arterial rupture in the time.
In these cases, the patient should be carried in an angiographic suite to have an arterial angiography with TAE. The advent of superselective microcatheters has made the detection of bleeding sources easier and TAE safer [45].
The most common embolic materials used are pushable microcoils and polyvinyl alcohol (PVA) particles. Different embolization strategies can be used depending on the location of the bleeding source:
If the bleeding source is in the main artery, microcoils can be first placed distal to the bleeding source (to prevent retrograde bleeding and to protect the thoracic wall from embolization). This is followed by placement of further microcoils proximal to the bleeding source. Eventually, final injection of PVA particles can accelerate thrombosis.
If the source is in a distal part of the artery and could not be reached by the microcatheter, embolization need to be performed by first injecting PVA particles of a medium size (250–500 μm) which can occlude distally to the bleeding source; then, by placing coils proximal to the bleeding afterwards.
These approaches reduce the risk of delayed failure of TAE due to perfusion from other feeding vessels that may be too small to detect during the first angiography [46].
Spinal cord ischemia is the only serious potential complication related to TAE and has been reported by several authors [47].
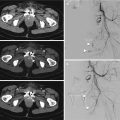
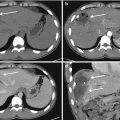
Nowadays, this adverse event is very rare because microcatheters can go beyond, with their tip, to the origin of medullary branches (Fig. 13.2).


Fig. 13.2
(a) CTA shows a small bleeding from a right intercostal artery. (b) DSA confirms the presence of a little contrast medium extravasation from the same vessel. (c) Embolization planning by the C-arm Cone Beam CT embolization software
13.3.5 Conclusions
Lesions of thoracic wall arteries are rare but potentially life threatening. Their management includes TEA or thoracotomy, but actually there aren’t standardized inclusion and exclusion criteria.
Some authors suggest that the endovascular treatment could be the most effective strategy to control hemorrhage, minimizing potential complications, so that TAE should always be considered before surgery in stable patients [48].
Whigham et al. [43] demonstrated a higher success of TAE with respect to surgery (91.6% vs. 66.0%) to stop the bleeding. Nonetheless, in most centers these traumas are still treated more frequently via thoracotomy.
13.4 Pleural and Airways Injuries in Trauma Patient
Airways trauma is a life-threatening condition which may be a result of blunt and penetrating injuries to the neck and chest [53]. Therefore, prompt diagnosis is mandatory for the survival of these patients, also because the presence of concomitant severe injuries and unspecific symptoms may delay the diagnosis and lead to early fatal outcome or late sequela such as airway stenosis and recurrent pulmonary infections [54]. The treatment of these patients provides an adequate ventilation and then the repair of the airways injury with a smaller impact on the respiratory function and the quality of life of the patients [55].
Blunt chest trauma causes an abrupt increase in intrathoracic airways pressure. If this happens against a closed glottis, the trachea and major bronchi can be injured secondary to the increased pressure.
The incidence of tracheobronchial injuries (TBI) among trauma patients with thoracic injuries, including those that died immediately, is estimated at 0.5–2% [56, 57]. The mortality from traumatic TBIs has decreased from 36% before 1950 to 9% in 2001 [53]. Laceration of the mediastinal pleura and/or bronchial injuries may allow air to enter in the pleural cavity; pneumothorax occurs in 17–70% of the patients with TBIs [58] and in 30–40% of all patients with a blunt chest trauma [59]. The most common cause is a rib fracture that lacerates the lung, but it also may be caused by rupture of a preexisting bleb at the time of impact [59]. Finally, hemothorax is seen in approximately 50% of patients who sustain blunt chest trauma [60]. Bleeding into the pleural space can originate from injury to the pleura, chest wall, lung, diaphragm, or mediastinum.
Concerning the approach to the patient with thoracic trauma, the airway in a neurologically intact patient is easily assessable: the injured patient who is alert and able to speak normally is maintaining a patent airway. However, especially in polytrauma patient, this must be carefully monitored as facial fractures with associated bleeding or edema, emesis, or foreign bodies can eventually compromise airway patency. The symptoms and signs of TBI depend on the site and the severity of the injury and most of them are not specific for this kind of injury.
Subcutaneous emphysema is the most common finding in TBI occurring in up to 87% of the patients [61]. Dyspnea, tachypnea, and respiratory distress are found in 59–100% of the patients [62], while hemoptysis can be seen in up to 74% of the cases [63].
Clinical signs of pneumothorax can be subtle and difficult to elicit in a patient who has multisystem trauma [60]. Detection of even a small, asymptomatic pneumothorax is important because up to one third can develop into a tension pneumothorax with potential cardiopulmonary decompensation [64].
The most common findings in chest X-rays are subcutaneous emphysema, pneumomediastinum, and pneumothorax.
Subcutaneous emphysema signs include: tracheal deformity, a defect in the tracheal contour, an endotracheal tube out of place, and the tube’s cuff over-inflated and protruding beyond the edge of the tracheal wall [65].
Radiographic signs of a pneumothorax can be subtle, and the appearance differs based on the patient position at the time that the radiograph was performed. In the supine position, air collects within the anterior costophrenic sulcus, which extends from the seventh costal cartilage to the 11th rib at the midaxillary line [64]. This appears radiographically as abnormal lucency in the lower chest or upper abdomen, an abnormally wide and deep costophrenic sulcus (the “deep sulcus” sign), a sharply outlined cardiac or diaphragmatic border, depression of the hemidiaphragm, or as a “double diaphragm” sign that is seen when air outlines the dome and anterior insertion of the diaphragm [66].
The appearance of hemothorax on a chest radiograph depends on the amount of blood that has collected in the pleural space and patient position. Plain radiography of the upright chest may be adequate to establish diagnosis by showing blunting at the costophrenic angle or an air–fluid interface if a hemopneumothorax is present. If the patient cannot be positioned upright, a supine chest radiograph may reveal apical capping of fluid surrounding the superior pole of the lung. In the acute trauma setting, the portable supine chest radiograph may be the first and only view available from which to make definitive decisions regarding therapy [60, 67]. When the size of a hemothorax reaches approximately 200 mL, an upright chest radiograph demonstrates blunting of the costophrenic angle. With progressive increase in size, a meniscus-sign will be seen: a concave upward sloping of fluid in the costophrenic angle. In contrast, a straight air–fluid level on the upright chest radiograph indicates a hemopneumothorax. A large hemothorax can opacify the hemithorax completely and cause contralateral shift of the mediastinum as the result of mass effect [68].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree