Thymic epithelial neoplasms are a group of malignant tumors that includes thymoma, thymic carcinoma, and thymic neuroendocrine tumors. Although several staging systems have been developed over the years for use with these cancers, they have been interpreted and implemented in a nonuniform manner. Recently, the International Association for the study of Lung Cancer and the International Thymic Malignancy Interest Group developed a tumor-node-metastasis staging system that has been universally accepted and correlates with patient survival and outcomes. Although pathologic staging is determined by histologic examination of the resected tumor, imaging plays an important role in clinical staging and is important for informing therapeutic decisions.
Key points
- •
Despite sharing a standardized staging system and many imaging characteristics, thymic epithelial neoplasms, including thymoma, thymic carcinoma, and thymic neuroendocrine tumors, differ from each other in several key clinical characteristics.
- •
The tumor-node-metastasis (TNM) staging system developed for thymic epithelial neoplasms correlates with patient survival and outcomes.
- •
The individual TNM descriptors are organized into specific stage groups.
- •
As part of the International Association for the study of Lung Cancer/International Thymic Malignancy Interest Group staging project, a lymph node map was developed that is similar to those developed for other neoplasms such as lung cancer.
- •
Although computed tomography (CT) is the imaging modality of choice for assessing the primary tumor and determining the T stage, MR imaging may be used to help assess for local invasion, and fluorodeoxyglucose PET/CT can be useful in identifying involved lymph nodes and metastases that may be overlooked on CT.
Introduction
Thymic epithelial neoplasms, a group of tumors including thymoma, thymic carcinoma, and thymic neuroendocrine cancers, are the most common primary malignancies of the prevascular mediastinum. As surgical resection is the cornerstone of treatment, accurate staging is necessary to differentiate between patients who are operative candidates and those who are not. Numerous staging systems have been developed over the years; however, their interpretation and implementation has been nonuniform. A partnership between the International Association for the study of Lung Cancer (IASLC) and the International Thymic Malignancy Interest Group (ITMIG) recently resulted in the creation of the first tumor-node-metastasis (TNM) staging system, which was adopted by the Union Internationale Contre le Cancer (UICC) and the American Joint Commission on Cancer (AJCC), effectively replacing previous staging schemes in January 2017. The objective of this article is to review the TNM staging system developed for thymic epithelial neoplasms and the role of imaging.
General considerations
Despite sharing the same staging system and many imaging characteristics, thymoma, thymic carcinoma, and carcinoid tumors differ from each other in several key clinical characteristics.
Thymoma, the most common thymic epithelial neoplasm, typically occurs in patients older than 40 years, peaking in the seventh decade, and affects men and women equally. , Most thymomas are solid neoplasms that are localized to the thymus but may exhibit aggressive behavior such as invasion of adjacent structures. Although involvement of the pleura and pericardium may occur, distant metastases are rare. With the widespread availability and increased use of computed tomography (CT), a greater number of thymomas are incidentally discovered while patients are asymptomatic. When patients present with clinical symptoms, such as dysphagia, diaphragm paralysis, or superior vena cava syndrome, these are usually related to local effects from compression or invasion of adjacent structures. Other patients may present with paraneoplastic syndromes, the most common of which is myasthenia gravis. Between 30% and 50% of patients with thymoma have myasthenia gravis, whereas only 10% to 15% of patients with myasthenia gravis have a thymoma. Additional paraneoplastic syndromes such as hypogammaglobulinemia and pure red cell aplasia are seen in 10% and 5% of patients with thymoma, respectively. Thymoma has also been associated with autoimmune disorders such as systemic lupus erythematosus, polymyositis, and myocarditis.
Thymic carcinoma comprises 20% of thymic epithelial neoplasms, with a mean age of 50 years at presentation. , It is more aggressive than thymoma and is more likely to result in local invasion and intrathoracic lymphadenopathy. At presentation, 50% to 65% of patients have distant metastases. , Symptoms usually relate to the intrathoracic local effects of the neoplasm, principally compression, and invasion of adjacent structures. In contrast to thymoma, paraneoplastic syndromes rarely accompany thymic carcinoma.
Thymic neuroendocrine neoplasms comprise only 2% to 5% of thymic epithelial neoplasms, most of which are carcinoid tumors. Thymic neuroendocrine tumors are more frequent in men, typically occurring in the fourth and fifth decades of life. Clinical symptoms reported by patients and imaging findings on cross-sectional imaging are similar to those encountered in thymic carcinoma. One-third of patients are asymptomatic, and the tumor may be discovered when imaging is performed as routine surveillance of patients with multiple endocrine neoplasia type 1, as they are predisposed to develop thymic carcinoids. Acromegaly, syndrome of inappropriate secretion of antidiuretic hormone, and carcinoid syndrome, although rare, are paraneoplastic syndromes associated with thymic neuroendocrine tumors. ,
Prognostication
Multiple key pieces of information related to thymic epithelial neoplasms affect patient prognosis, including anatomic spread of disease, histologic classification, age, and functional status. The histologic classification of thymic epithelial neoplasms was most recently updated by the World Health Organization (WHO) Consensus Committee in 2015. Thymomas are classified into 5 separate histologic subtypes—A, AB, B1, B2, B3—based on the morphology of the neoplastic epithelial cells together with the lymphocyte. Thymic carcinomas are divided into multiple histologic subtypes including adenocarcinoma, squamous, basaloid, mucoepidermoid, lymphoepithelioma-like, clear cell, and sarcomatoid carcinomas. Thymic neuroendocrine tumors are divided into carcinoid, large cell carcinomas, and small cell carcinomas. Although thymic carcinoma and thymic neuroendocrine neoplasms are associated with a poorer prognosis compared with thymoma, the different subtypes of thymoma have little practical clinical use. The WHO histologic classification lacks inter- and intraobserver reproducibility and clinical predictive value. , In addition, multiple WHO subtypes are often present in the same tumor, which makes classification more challenging especially in needle biopsy specimen where the predominant tumor subtype may not be sampled. Management decisions rest primarily on the stage of disease and the completeness of resection, both of which were repeatedly found to correlate with prognosis.
Multiple anatomic staging schemes have been used over the years, all based on small series from single institutions. Before 2017, the most widely used staging systems included the Masaoka system, proposed in 1981, based on 91 patients, or and its variant, the Masaoka-Koga system, proposed in 1994, based on 79 patients. The Masaoka-Koga staging is based on the gross and microscopic properties of the tumor. Stage I tumors are characterized by complete encapsulation; stage II by microscopic invasion through the capsule (IIa) or macroscopic invasion into surrounding fat (IIb); stage III by invasion into any neighboring organs such as the pericardium, great vessels, trachea, esophagus, or lung; and stage IV by pleural or pericardial metastases (IVa) or hematogenous or lymphatic metastases (IVb).
Several limitations of the Masaoka-based staging systems have been described. The reliance on a capsule for staging was difficult, as not all thymomas have a complete capsule. Masaoka stage III included involvement of many organs; however, the staging was based on such a small number of patients that there was insufficient statistical power to address the nuances of the stage. The TNM staging system developed by the IASLC and ITMIG used a retrospective database of 10,808 cases gathered from 105 institutions worldwide, which correlates with overall survival.
Tumor-necrosis-metastasis descriptors
Primary Tumor
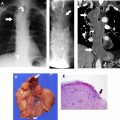
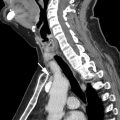
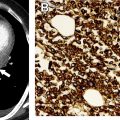
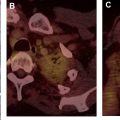
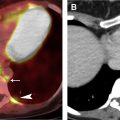
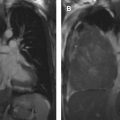
The tumor (T) descriptor is determined by the presence and extent of local tumor invasion ( Table 1 ). T1 describes encapsulated or unencapsulated tumors with or without extension into the adjacent prevascular (perithymic) mediastinal fat. The T1 category is further divided into T1a with no mediastinal pleural involvement or T1b with direct invasion of the mediastinal pleura ( Fig. 1 ). T2 describes lesions with direct invasion of the pericardium ( Fig. 2 ). T3 is characterized by tumor involvement of the lung, brachiocephalic vein, superior vena cava, phrenic nerve, chest wall, or extrapericardial pulmonary artery or veins ( Fig. 3 ). Finally, T4 tumors include those that invade the aorta (ascending, arch, or descending), aortic arch vessels (brachiocephalic, carotid, and subclavian arteries), intrapericardial pulmonary artery, myocardium, trachea, or the esophagus ( Fig. 4 ).
| T | T1a | Encapsulated or unencapsulated, with or without extension into mediastinal fat |
| T1b | Extension into mediastinal pleura | |
| T2 | Involvement of pericardium | |
| T3 | Involvement of lung, brachiocephalic vein, superior vena cava, chest wall, phrenic nerve, hilar (extrapericardial) pulmonary vessels | |
| T4 | Involvement of aorta, arch vessels, main pulmonary artery, myocardium, trachea, or esophagus | |
| N | N0 | No lymph node metastasis |
| N1 | Involvement of anterior (perithymic) lymph nodes | |
| N2 | Involvement of deep intrathoracic or cervical lymph nodes | |
| M | M0 | No metastasis |
| M1a | Pleural or pericardial metastatic nodules | |
| M1b | Pulmonary intraparenchymal metastatic nodule or distant organ metastasis |




Regional Lymph Nodes
The lymph node (N) descriptor is determined by the presence or absence of intrathoracic lymph node involvement. The N stage defines lymph node regions as defined by the IASLC/ITMIG project (KP) . N0 describes the absence of lymph node metastasis. N1 and N2 represent involved lymph nodes located in the prevascular and deep spaces of the mediastinum, respectively, as outlined in the specific lymph node map created for use with thymic epithelial neoplasms ( Fig. 5 ). N1 node includes prevascular mediastinal and perithymic lymph nodes, and N2 disease describes deep intrathoracic and cervical lymph nodes, including tracheobronchial and aortopulmonary window, internal mammary, deep cervical, and supraclavicular lymph nodes (see Table 1 ).