Thymic epithelial neoplasms, as classified by the World Health Organization, include thymoma, thymic carcinoma, and thymic carcinoid. They are a rare group of tumors and are often diagnosed incidentally in the work-up of parathymic syndrome, such as myasthenia gravis, or when mass effect or local invasion causes other symptoms. In each of these scenarios, understanding the radiologic-pathologic relationship of these tumors allows clinical imagers to contribute meaningfully to management decisions and overall patient care. Integrating important imaging features, such as local invasion, and pathologic features, such as necrosis and immunohistochemistry, ensures a meaningful contribution by clinical imagers to the care team.
Key points
- •
Thymic epithelial neoplasms, including thymoma, thymic carcinoma, and thymic carcinoid, are a rare group of tumors occurring in the anterior or prevascular mediastinum.
- •
Clinical presentations and syndromes associated with thymic epithelial neoplasms are important diagnostic clues to consider, and these clinical factors may ultimately guide management.
- •
The imaging features of these entities may overlap and an understanding of the radiologic-pathologic relationship helps clinical imagers arrive at the correct diagnosis and guide appropriate therapy.
Introduction
Thymic epithelial neoplasms, as classified by the World Health Organization (WHO), include thymoma, thymic carcinoma, and thymic carcinoid. They are a rare group of tumors, and may be diagnosed incidentally, in the work-up of a parathymic syndrome, such as myasthenia gravis, or when mass effect or local invasion causes other symptoms. In each of these scenarios, understanding the radiologic-pathologic relationship of these neoplasms allows clinical imagers to contribute meaningfully to management decisions and overall patient care.
Thymoma
Clinical Considerations
In adults older than the age of 40, thymomas are the most common neoplasm in prevascular mediastinum. They are uncommon in children and adolescents. Thymomas account for approximately 80% of all thymic epithelial tumors and have no gender predilection. Approximately one-third of patients are asymptomatic with many thymomas identified incidentally on thoracic imaging for other indications. However, depending on the size and location, thymomas can cause chest pain, dyspnea, cough, or phrenic nerve palsy. If the mass obstructs the superior vena cava, patients may develop symptoms, such as arm or facial swelling. The histologic components of thymomas also predispose patients to paraneoplastic syndromes, many of which are a result of autoimmune disorders and can result in a wide range of presenting symptomatology based on the associated abnormalities.
The thymus plays a key role in the developing immune system and is required for normal maturation of T lymphocytes. This maturation process relies on normal thymic architecture. Although most thymomas occur in adults after the normal thymus has involuted, they can produce and release immature T lymphocytes into systemic circulation. Without the normal architecture of the thymus to filter out abnormal or self-reactive T lymphocytes, these immature cells may result in autoimmune disorders. The amount and type of lymphocytes produced varies by subtype of thymoma.
The most frequent paraneoplastic autoimmune syndrome associated with thymoma is myasthenia gravis ( Table 1 ). One-third to one-half of patients with thymoma have myasthenia gravis and in most cases there are abnormal antibodies directed toward acetylcholine receptors. Conversely, only 10% to 20% of patients with myasthenia gravis have a thymoma. Regardless, patients who present with myasthenia gravis should be screened for thymoma at diagnosis. Other, less common paraneoplastic syndromes associated with thymoma include hypogammaglobulinemia in 5% to 20%, pure red cell aplasia in approximately 4%, and neuromyotonia (Isaac syndrome) in 3%.
| Subtypes | 5-y Survival | Histopathology | Imaging | Associated Syndromes |
|---|---|---|---|---|
| Thymomas b (>70%) adults 50–60 y with wide range | ||||
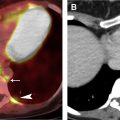
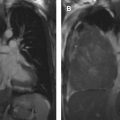
| Type A | 100% | Spindloid epithelial cells with rare to no lymphocytes | CT (commonly): Homogenously enhancing, unilateral lobulated mass with smooth margins and fibrous capsule; completely or partially outlined by fat CT (rarely): Heterogeneously enhancing mass arising anywhere from lower neck to cardiophrenic borders with loculations; cystic components; curvilinear or punctate calcifications; irregular margins; local invasion; mediastinal adenopathy; and metastases (<5%) to pleura/pericardium MR imaging: Intermediate T1 and hyperintense T2, with hypointense internal fibrous septa and fibrous capsule; CSR c >1.0 FDG PET/CT: Low or variable FDG uptake; SUV max d <11.6 SSTR (+) | Myasthenia gravis (35%); hypogammaglobulinemia (5%–20%); red cell aplasia (4%); neuromyotonia (3%) |
| Type AB | 100% | Spindloid epithelial cells admixed with lymphocyte-rich foci, either diffusely or in discrete nodules | ||
| Type B1 | 95% | Polygonal epithelial cells forming anastomosing network obscured by normal thymic architecture with diffuse lymphocytes | ||
| Type B2 | 95% | Polygonal epithelial cells in clusters with diffuse lymphocyte-rich background; possible palisading of tumor cells along perivascular spaces; epithelial cell nuclei large and vesicular with prominent nucleoli | ||
| Type B3 | 92% | Polygonal epithelial cells with mild-to-moderate atypia forming confluent sheets with rare lymphocytes; epithelial cell nuclei moderate in size | ||
| Thymic carcinoma (20%) adults older than 50 y | ||||
| Type C | 38% | Clear-cut cytologic atypia with cytoarchitecture dissimilar to normal thymus; range of morphologic subtypes f | CT: Heterogeneously enhancing mass with loculations; calcifications (10%–61%); irregular margins; local invasion; and distant metastases (50%–65%) to brain, bone, lung, and liver MR imaging: Solid components hyperintense on T1 and T2; CSR >1.0; ADC e values <1.56 × 10 −3 mm /s FDG PET/CT: High FDG uptake SUV max >11.6 SSTR (+) | Rarely: Myasthenia gravis; red cell aplasia; hypogammaglobulinemia |
| Thymic carcinoid tumor (2%–5%) Male (3:1) adults 40–60 y with wide range | ||||
| Typical | 50%–100% | Predominantly or exclusively neuroendocrine cells marked by diffuse expression of neuroendocrine markers g in 50% of tumor cells; low (typical) or intermediate (atypical) grade; range of histologic subtypes h | CT: Heterogeneously enhancing unencapsulated mass with possible calcifications; irregular margins; local invasion; and distant metastases (20%–40%) to skin, adrenal glands, bone, lung, pleura, brain, or kidney. MR imaging: Heterogenous hyperintensity on T2 FDG PET/CT: High FDG uptake SSTR (++) MIBG (+) | Functional activity (33%–50%) resulting in endocrinopathies (Cushing syndrome in 40%; SIADH; carcinoid syndrome in 2%) Rarely: MEN type 1 (25%) and 2 |
| Atypical | 20%–80% | |||
a Excluding rare neoplasms, including high-grade thymic neuroendocrine tumors (small cell carcinoma; large cell neuroendocrine carcinoma) and thymic teratoma.
b ( A ) Excluding rare types of thymomas, including micronodular thymoma with lymphoid stroma, metaplastic thymoma, microscopic thymoma, sclerosing thymoma, lipofibroadenoma, and atypical type A variant. ( B ) More than 50% of thymomas are classified as multiple subtypes. Estimated prevalence of each subtype: type A (4%–7%), type AB (28%–34%), type B1 (9%–20%), type B2 (20%–36%), and type B3 (10%–14%).
c Chemical shift MR imaging may distinguish benign (CSR <0.7) from neoplastic (CSR >1.0) tissue. CSR is derived from dual-echo, single breath-hold gradient echo T1-weighted imaging with in-phase (IP) and opposed-phase (OP) images. CSR = [(thymus OP/muscle OP)/(thymus IP/muscle IP)].
d 1 FDG PET/CT maximum SUV may distinguish thymoma (SUV max <11.6) from thymic carcinoma (SUV max >11.6).
e ADC values >1.56 × 10 —3 mm 2 /s predict that a tumor is benign with 94% specificity, and higher ADC values correspond to well-differentiated tumors.
f Subtypes include squamous (most common), mucoepidermoid, lymphoepithelioma-like, clear cell, basaloid, adenocarcinoma, papillary, rhabdoid, hepatoid, rhabdomyomatous, sarcomatoid, and undifferentiated/anaplastic.
g Neuroendocrine markers include synaptophysin, chromogranin, CD56, and neuron-specific endolase.
h Subtypes include classic carcinoid, spindle cell, pigmented, oncocytic/oxyphilic, mucinous, angiomatoid, carcinoid with sarcomatous change, and carcinoid with amyloid.
Numerous categorization systems have been developed for thymoma, which has resulted in significant interobserver variability regarding how these tumors have been classified, staged, and treated over time. The Masaoka clinical staging system, first proposed in 1981, is an excellent predictor of prognosis for thymoma but is not as well suited for thymic carcinomas. , Subsequently, a preliminary tumor-node-metastasis (TNM) classification system was proposed in 1991 and a modified classification by Koga and colleagues in 1994. , The Masaoka-Koga modified classification system is characterized by the following features , :
- •
Stage I: Grossly and microscopically completely encapsulated tumor
- •
Stage IIa: Microscopic transcapsular invasion
- •
Stage IIb: Macroscopic invasion into thymic or surrounding fatty tissue, or grossly adherent to but not breaking through mediastinal pleura or pericardium
- •
Stage III: Macroscopic invasion into neighboring organ, such as the great vessels, pericardium, or lung
- •
Stage IVa: Pleural or pericardial dissemination
- •
Stage IVb: Lymphogenous or hematogenous metastasis
In the most recent 2017 American Joint Committee on Cancer TNM (eighth edition) system for thymomas, staging is based on local invasion, lymph node involvement, and metastasis. The T categories are determined by the presence or absence of invasion and, if present, what surrounding structures are involved. The incidence of lymph node involvement or distant metastasis in thymoma is less than 5%. When lymph node involvement does occur from thymoma, it is localized to the prevascular mediastinum 90% of the time. The currently accepted method for describing lymph node involvement from thymoma is separated into two groups, anterior and deep, according to the proposed lymph node mapping schema developed by the ITMIG/IASLC project. When distant metastasis is present, it is grouped into pleural/pericardial or distant.
Pathologic Features
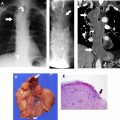
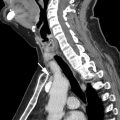
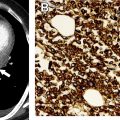
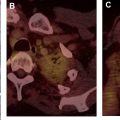
The most widely used histologic classification system is the WHO fourth edition published in 2015 by Travis and colleagues, which is summarized next. There are five main subtypes of thymomas: type A, AB, B1, B2, and B3. Other recognized subtypes of thymomas include micronodular thymoma with lymphoid stroma, metaplastic thymoma, microscopic thymoma, sclerosing thymoma, and lipofibroadenoma. Because thymomas are frequently composed of several subtypes, it is now recommended to include all identified subtypes in the pathologic report (except for type AB thymomas, which are already a combination of types A and B by definition) in order of predominance by 10% increments. The 2015 WHO classification system also includes immunohistochemistry, which may help differentiate thymomas with otherwise ambiguous histology ( Figs. 1–3 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree