Study
Enrollment
Number of patients
Approach
Device
Procedural success
30-day mortality
I-REVIVE/RECAST [6]
2003–2005
26
Transseptal
Edwards SAPIEN
85 % (22/26)
16.7 % (6/36)
7
TF
Edwards SAPIEN
57 % (4/7)
Grube et al. [9]
2005–2007
86
TF
CoreValve
74 % (64/86)
11.6 % (10/86)
TRAVERCE [26]
2006–2008
168
TA
Edwards SAPIEN
95.8 % (161/168)
14.9 % (25/168)
2006–2008
40
TA
Edwards SAPIEN
100 % (40/40)
12.5 % (7/40)
2005–2006
55
TF
Edwards SAPIEN
87 % (48/55)
7.3 % (4/55)
Registries (Table 2.2)
Table 2.2
Clinical outcomes after TAVR according to access site and device type
Major published data | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Authors | Type of study | Number of patients | STS (%) | Logistic EuroScore (%) | Follow-up (months) | Procedural success rate (%) | Mortality 30-day (%) | Mortality 1-year (%) | Major access complications 30-day (%) | Stroke 30-day (%) | Need for new PPM (%) |
Edwards SAPIEN: TF | |||||||||||
Lefevre et al. [12] | Registry | 61 | 11.3 | 25.7 | 12 | 95.4 | 8.2 | 21.3 | 16.4 | 3.3 | 1.8 |
Eltchaninoff et al. [8] | Registry | 95 | 17.4 | 25.6 | 1 | 98.3a | 8.4 | – | 6.3 | 4.2 | 5.3 |
Himbert et al. [11] | Registry | 51 | 15.0 | 25.0 | 12 | 90.0 | 8.0b | 19.0 | 12.0 | 6.0 | 6.0 |
Rodes-Cabau et al. [15] | Registry | 162 | 9.0 | – | 24 | 90.5 | 9.5 | 25.0 | 13.1 | 3.0 | 3.6 |
Registry | 463 | – | 14.5 | 1 | 95.2 | 6.3 | 18.9 | 22.9 | 2.4 | 6.7 | |
Leon et al. [20] | RCT | 179 | 11.2 | 26.4 | 12 | – | 5.0c | 30.7c | 16.2 | 6.7d | 3.4 |
Bosmans et al. [4] | Registry | 99 | – | 29.0 | 12 | 97.0 | 6.0 | 18.0 | – | 2.0 | 4.0 |
Smith et al. [21] | RCT | 244 | 11.7 | 29.1 | 12 | – | 3.3c | 22.2c | 14.0 | 3.7d | 3.7 |
Edwards SAPIEN: TA | |||||||||||
Walther et al. [26] | Feasibility study | 168 | – | 27.0 | 12 | 95.8 | 15.0 | 37.0 | 1.2 | 2.0 | 2.3 |
Svensson et al. [25] | Feasibility study | 40 | 13.4 | 35.5 | 6 | 87.5 | 17.5 | – | – | 5.0 | – |
Lefevre et al. [12] | Registry | 69 | 11.3 | 33.8 | 12 | 96.4 | 18.8 | 50.7 | 5.8e | 1.5 | 3.8 |
Eltchaninoff et al. [8] | Registry | 71 | 18.4 | 26.8 | 1 | 98.3a | 16.9 | – | 5.6f | 2.8 | 5.6 |
Himbert et al. [11] | Registry | 24 | 18.0 | 28.0 | 12 | 100 | 16.0b | 26.0 | 8.0 | 0 | 4.0 |
Rodes-Cabau et al. [15] | Registry | 177 | 10.5 | – | 1 | 96.1 | 11.3 | 22.0 | 13.0f | 1.7 | 6.2 |
Registry | 575 | – | 16.3 | 1 | 95.7 | 10.3 | 27.9 | 4.7 | 2.6 | 7.3 | |
Bosmans et al. [4] | Registry | 88 | – | 33.0 | 12 | 97.0 | 14.0 | 37.0 | – | 8.0 | 6.0 |
Smith et al. [21] | RCT | 104 | 11.8 | 29.8 | 12 | – | 3.8c | 29.0c | 3.8 | 6.8 | 3.9 |
D’Onofrio et al. [7] | Registry | 504 | 11.0 | 26.3 | 24 | 99.0 | 8.3 | 18.8 | – | 3.0 | 5.4 |
Medtronic CoreValve™: TF | |||||||||||
Tamburino et al. [16] | Registry | 663 | – | 23.0 | 12 | 98.0 | 5.4 | 15.0 | 2.0 | 2.5g | 17.4 |
Bosmans et al. [4] | Registry | 133 | – | 25.0 | 12 | 98.0 | 11.0 | 22.0 | – | 4.0 | 22.0 |
Grube et al. [10] | Registry | 86 | – | 21.6 | 1 | 88.0 | 12.0 | – | – | 10.0 | – |
Piazza et al. [14] | Registry | 646 | – | 23.1 | 1 | 97.2 | 8.0 | – | 1.9 | 1.9 | 9.3 |
Eltchaninoff et al. [8] | Registry | 66 | 21.3 | 24.7 | 1 | 98.3a | 15.1 | – | 7.5 | 4.5 | 25.7 |
Petronio et al. [13] | Registry | 460 | – | 19.4 | 6 | 98.4 | 6.1 | 11.4 | 2.0 | 1.7 | 16.1 |
Buellesfeld et al. [5] | Registry | 126h | – | 23.0 | 24 | 72.6 | 15.2 | 28.1 | – | 9.6 | 26.2 |
Medtronic CoreValve™: SC | |||||||||||
Eltchaninoff et al. [8] | Registry | 12 | 21.0 | 24.6 | 1 | 98.3a | 8.3 | – | 8.3 | 0 | 25.0 |
Bosmans et al. [4] | Registry | 8 | – | 25.0 | 12 | 98.0 | 11.0 | 0 | – | 4.0 | 22.0 |
Petronio et al. [13] | Registry | 54 | – | 25.3 | 6 | 100 | 0 | 6.7 | 0 | 1.9 | 18.5 |
Zahn et al. [29]i | Registry | 697 | – | 20.5 | 1 | 98.4 | 12.4 | – | 19.5 | 2.8 | 39.3j |
Edwards Registries
Several large European and Canadian registries have been published, showing excellent short- and midterm results after TAVR using both the TF and TA devices [12, 15]. The largest registry reported to date is the SOURCE (SAPIEN Aortic Bioprosthesis European Outcome) registry [17, 18]. Overall, 1,038 patients were enrolled at 32 European centers and were treated with either a TF (n = 463) or TA approach (n = 575). Patients treated by TA had more comorbidities at baseline than TF patients, resulting in a significantly higher EuroSCORE (29.1 % vs. 25.7 %; P < 0.001). Procedural success was 95.2 and 92.7 %, and 30-day mortality was 6.3 and 10.3 % in the TF and TA populations, respectively. The major limitations of this registry were that more than 70 % of the enrolling centers had no prior experience with TAVR and all adverse events were site-reported without core lab analysis. In early 2011, 1-year results were published, demonstrating a 1-year survival of 76.1 % overall, 72.1 % for TA and 81.1 % for TF patients. Among the surviving patients, 73.5 % were NYHA class I or II [17].
CoreValve™ Registries
A number of large dedicated CoreValve registries have been reported [14, 16]. Promising 3-year results were recently reported by Ussia et al. [27], and although not yet published, the results of the ADVANCE CoreValve™ registry were presented recently [28]. ADVANCE represents a 100 % monitored “real-world” experience, with a core laboratory and an independent clinical event committee adjudicating events. The registry included 1,015 patients from 44 experienced (>40 prior procedures) centers between March 2010 and July 2011. The mean logistic EuroSCORE was 19.2 %. At 30 days and 6 months, the rate of all-cause mortality was 4.5 and 12.8 %, respectively, with cardiac mortality of 3.4 and 8.4 %, respectively. ADVANCE provides insights into contemporary TAVR data of experienced operators and is a benchmark for comparing outcomes.
Mixed National Registries
In 2011, results from four mixed CoreValve™ and Edwards European national registries have been reported, mostly using the TF and TA routes (Table 2.2) [4, 8, 29, 30]. Overall, patients included in these registries were at high risk according to surgical risk models, mean EuroSCORE 18–30 %. These registries showed 1-year survival rates ranging between 71.9 and 81.6 %. The UK registry reported the longest follow-up; survival was 73.7 % at 2 years [30]. Several of these national initiatives performed access-route comparisons and reported that survival was generally higher in patients treated through the TF route [4, 30]. However, it should be noted that a transfemoral-first approach is often advocated, which may introduce selection bias and an unequal comparison between the two access routes [31].
Recently, the largest registry to date was reported by the FRANCE 2 investigators [32]. They included 3,195 patients treated between January 2010 and December 2011 at 34 centers. The registry reflects contemporary real-life use of available TAVR devices in patients at high surgical risk; the Edwards SAPIEN and the Medtronic CoreValve™ devices were used in, respectively, 66.9 and 33.1 %. The transfemoral approach was most widely used (74.6 %), followed by transapical (17.8 %), subclavian (5.8 %), and 1.8 % by a non-femoral non-apical approach. Procedural success rate was 96.9 % and 1-year survival in patients was 76.0 %.
Randomized Trials
Completed Trials
While registry reports are of crucial value to assess “real-world” use of TAVR, more rigorous assessments are available from the first multicenter, randomized clinical PARTNER trials (Placement of Aortic Transcatheter Valves; ClinicalTrials.gov Identifier: NCT00530894) (Fig. 2.1) [20, 21]. As the first of two parallel trials completed, the results of PARTNER IB showed that TF TAVR was superior to standard therapy in patients not deemed candidates for surgery [20]. The primary endpoint of all-cause mortality was markedly reduced by 46 % (P < 0.001). Recently reported 2-years outcomes showed continued encouraging results (Fig. 2.2a) [33]. At 2 years, the primary endpoint of all-cause mortality was reduced from 67.6 % in the standard treatment arm to 43.3 % in the TAVR arm (P < 0.001).
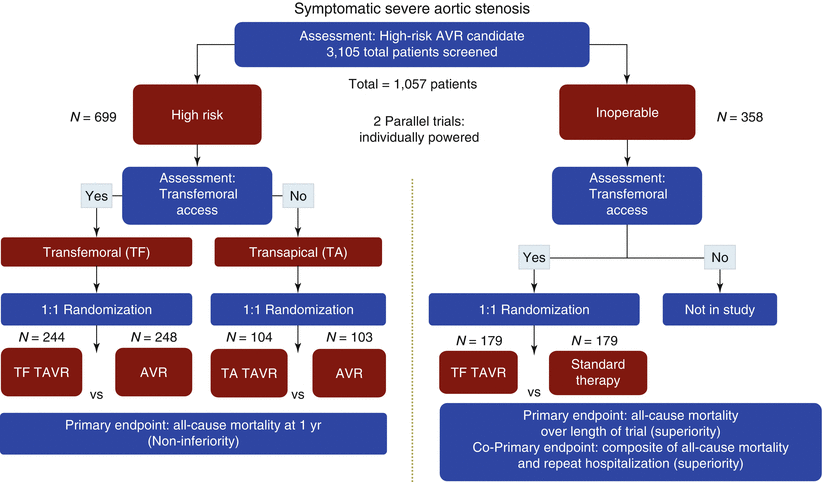
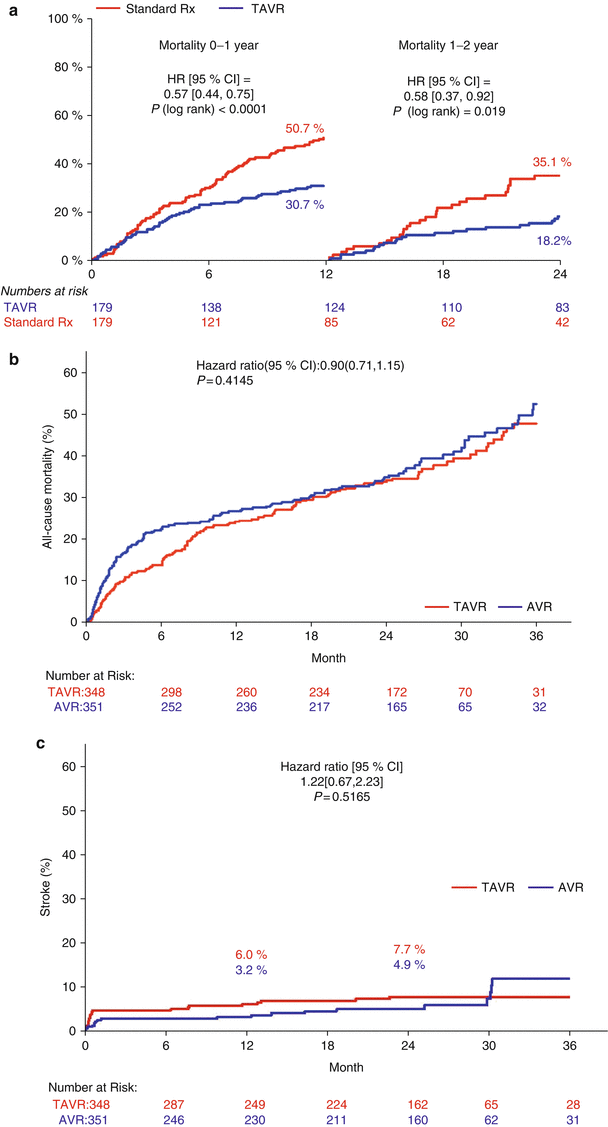

Fig. 2.1
PARTNER trial I design

Fig. 2.2
(a) A 2-year with 1-year landmark analysis of all-cause mortality Kaplan-Meier curve in PARTNER trial cohort 1B (Reprinted with permission from Leon and colleagues and Makkar and colleagues [20, 33]). (b) A 2-year all-cause mortality Kaplan-Meier curve in PARTNER trial cohort 1A (Adapted with permission from Kodali and colleagues [34]). (c) A 2-year stroke Kaplan-Meier curve in PARTNER trial cohort 1A (Adapted with permission from Kodali and colleagues [34])
The PARTNER cohort IA compared TAVR to SAVR and met its non-inferiority endpoint: the all-cause 1-year mortality in the TAVR group was non-inferior to the SAVR group (24.2 % vs. 26.8 %; P = 0.44; P = 0.001 for non-inferiority) [21]. Some concerns were raised with regard to early neurologic events that were higher by TAVR as compared to SAVR at 30 days (5.5 % vs. 2.4 %; P = 0.04) and 1 year (8.3 % vs. 4.3 %; P = 0.04). Although the recently published 2-year results revealed that stroke rates were similar for TAVR and SAVR during 1 and 2 years with a hazard ratio of 1.22 (95 % CI 0.67–2.23, P = 0.52), the issue of stroke warrants further investigation and should not be underestimated (Fig. 2.2b, c) [34]. The rate of the composite of all-cause death and stroke was encouragingly similar after TAVR (37.1 %) and SAVR (36.4 %) at 2 years (P = 0.85).
Ongoing Trials
In the USA, a randomized trial is currently ongoing to evaluate the safety and efficacy of the Medtronic CoreValve™ in the treatment of severe symptomatic AS in patients at high or extreme risk for SAVR (ClinicalTrials.gov Identifier: NCT01240902). The trial consists of two arms. Patients in a high-risk arm will be randomized between SAVR and TAVR; the primary endpoint consists of all-cause mortality at 1 year. An extreme risk arm will function as an observational arm in which a composite of all-cause mortality and major stroke is the primary endpoint.
As a sequel to the PARTNER I trial, a second randomized trial (PARTNER II) is currently ongoing. It was designed to investigate the performance and outcomes after TAVR with the next-generation Edwards SAPIEN XT valve, model 9300TFX, as well as the new low-profile 18-Fr NovaFlex™ delivery catheter in patients deemed non-operable (ClinicalTrials.gov Identifier: NCT01314313) (Fig. 2.3). Given the results of the control arm in PARTNER IB, it has been judged that a study comparing TAVR against a “medical management” control group is no longer ethical [35]. Consequently, an “old device” versus “new device” non-inferiority trial was designed. Enrolment was initiated in January 2011, and it is anticipated that primary endpoint results will be published mid-2013.
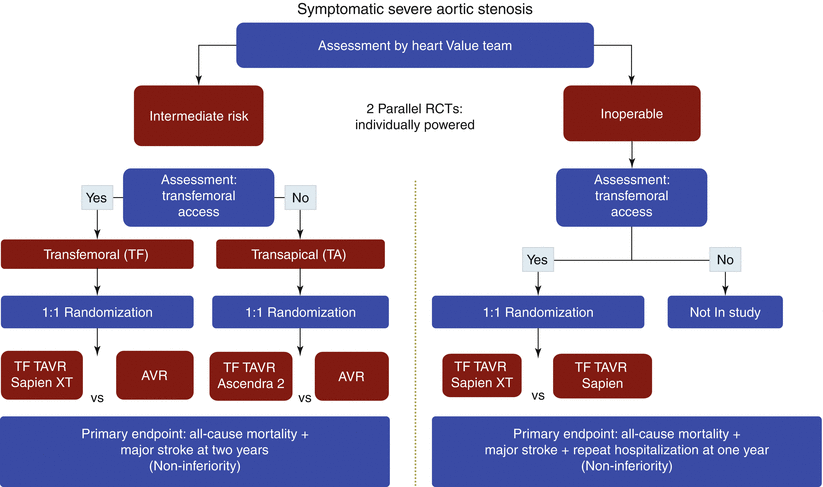

Fig. 2.3
PARTNER trial 2 design
In Denmark, a phase 2 randomized trial evaluating TAVR in patients ≥70 years of age started enrollment in December 2009 (ClinicalTrials.gov Identifier: NCT01057173). The trial will randomize a total of 280 patients to TAVR (n = 140) and SAVR (n = 140). The primary endpoint is the composite of all-cause death, myocardial infarction, and stroke at 1 year and is scheduled to be completed late 2013.
In an attempt to expand the indication of TAVR to lower-risk patients, the PARTNER IIA trial will be randomizing patients between TAVR with the SAPIEN XT valve and SAVR in intermediate-risk patients (ClinicalTrials.gov Identifier: NCT01314313) (Fig. 2.3). Similarly, the prospective randomized, international SURTAVR trial will randomize 1,900 intermediate-risk patients between TAVR with the Medtronic CoreValve™ and SAVR at approximately 80 centers throughout the USA, Canada, Europe, and Australia (ClinicalTrials.gov Identifier: NCT01586910).
Alternative Access Sites
Like the TA approach, a subclavian approach allows patients with unfavorable iliofemoral anatomy or extensive disease to be treated with TAVR. Petronio et al. reported a series of 54 patients, showing a procedural success rate of 100 %, a procedural mortality of 0 %, a 30-day mortality of 0 %, and a 6-month mortality of 9.4 % [13]. No specific vascular complications for subclavian access were reported. The subclavian approach is usually performed with the self-expanding CoreValve™ system and can be fully percutaneous [36].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree