Variceal bleeding is a major cause of death in portal hypertension. In patients with varices, the risk of a first hemorrhage is around 4% per year. Risk factors include size of varices, Child-Pugh class B or C, and hepatic vein pressure gradient (HVPG) greater than 12 mmHg. HVPG is measured using a transjugular catheter, comparing pressure when the catheter tip is wedged and free within the vein, and reflects the portal-to-systemic pressure gradient. Esophageal varices account for 70% of bleeding, and most of the remainder are gastric variceal hemorrhages. Mortality within 6 weeks of bleeding is about 30%. It is most often related to uncontrolled bleeding or early rebleeding. Other important causes of death after variceal bleeding are liver failure, multiorgan failure, and sepsis. Without preventive treatment, rebleeding occurs within 2 years in up to 65% of patients, and mortality from rebleeding is 33%.1 Ascites in patients with cirrhosis and portal hypertension is a result of multiple factors. Elevated venous pressure in the gut causes low protein fluid transudate into the peritoneal cavity. Elevated sinusoidal pressure causes high protein transudate from the liver surface. Renal effects of cirrhosis result in sodium and water retention. Reduced albumin contributes to extravascular fluid retention. Ascites causes significant impairment of quality of life. Frequent percutaneous drainage procedures may be required. Complications include spontaneous bacterial peritonitis and hepatorenal syndrome. TIPS is most effective in treatment of ascites when the HVPG is high.2 Endoscopic banding is effective in controlling acute bleeding in 80% to 90% of esophageal varices. Gastric varices are more difficult to control but may respond to endoscopic glue injection. Ectopic varices are not treatable endoscopically. Emergency TIPS is indicated when endoscopic therapy fails to control esophageal or gastric variceal bleeding and as a first-line therapy for bleeding ectopic varices. Outcomes are best when TIPS is performed early, ideally within 24 hours of presentation.3,4 TIPS is also used for prevention of rebleeding. Recurrence of bleeding after successful endoscopic control is a common problem, with high morbidity, mortality, and cost. Even with preventive medical therapy, rebleeding occurs in 25% to 33% of patients over 18 months.5 A recent randomized controlled trial identified patients with high risk of rebleed and compared the outcome of endoscopic banding plus TIPS with banding alone. The study demonstrated a marked reduction in rebleeding and improved patient survival at 1 year.4 The authors identified the timing of TIPS within 72 hours of endoscopy as a critical factor in producing an improved clinical outcome. Gastric varices have a greater tendency to rebleed acutely after successful TIPS. Rebleeding can occur despite reducing the portal systemic pressure gradient to less than 12 mmHg. For this reason, transvenous embolization and sclerosis of varices should be performed routinely during TIPS for bleeding gastric varices.6 Transjugular intrahepatic portosystemic shunting is effective in reducing ascites in patients with portal hypertension. In addition to symptomatic control, potential benefits are reduced risk of hepatorenal syndrome and spontaneous bacterial peritonitis. However, its role is limited by the risk of developing the complications of encephalopathy and progressive liver failure. Results of trials and meta-analyses are mixed when assessing benefits to survival and quality of life.7–11 Improvement in control of ascites can be expected in approximately two thirds of patients. New or worsened encephalopathy is seen in 32%. This can usually be controlled with medication but may require a shunt-reducing stent or shunt occlusion.12 Six-month mortality is around 36%.7 Poor outcome is more likely in those with a bilirubin level over 3 mg/dL, creatinine level over 1.5 mg/dL, and age older than 60.9 Improved ascites control is more likely in those with a higher portosystemic pressure gradient (mean 21 mmHg vs. 15 mmHg).10 Although no randomized studies have been performed, patients treated with TIPS have a good long-term survival compared with historical series and predicted survival based on biochemical and clinical parameters at presentation.13,14 Control of symptoms and prevention of disease progression means these patients can delay or avoid the need for liver transplantation.15 With some patients’ liver anatomy, it may be feasible or preferred to place percutaneous portosystemic shunts directly from the inferior vena cava (IVC) to the portal vein or potentially between other portal and systemic veins with appropriate anatomy. The direct intrahepatic portosystemic shunt (DIPS) extends from the intrahepatic IVC through a short parenchymal track in the caudate lobe of the liver into the portal vein. Stented to 8 mm with a covered balloon-expandable stent, it provides a short low-resistance shunt with minimal liver trauma and low risk of restenosis.16 In patients with predicted poor outcome from TIPS, bleeding can be controlled using alternative interventional techniques, either BRTO17 or transhepatic transportal injection of sclerosant, with or without coil embolization. Although these techniques are uncommon in Western countries, they are widely practiced in some Japanese and Chinese centers to the point where they are preferred to TIPS, particularly in the treatment of gastric variceal bleeding. Published results suggest these techniques may result in improved survival with lower rates of encephalopathy and rebleeding. Relative contraindications to TIPS include: • Cardiac failure, elevated right-sided heart pressure, and pulmonary hypertension • Rapidly progressive liver failure • Severe uncorrectable coagulopathy • Unrelieved biliary obstruction • Extensive primary or metastatic hepatic malignancy Polycystic liver disease has previously been considered a contraindication, but TIPS has been performed safely in these patients with the use of bare metal stents. It is likely to be safe with covered stents, although this remains to be tested.18 Coagulopathy, shock, and sepsis are relative contraindications, but it may still be appropriate to perform an urgent TIPS procedure while these are being corrected. Other relative contraindications are predictors of poor prognosis after TIPS. The most accurate prediction of post-TIPS mortality is the Model for End-Stage Liver Disease (MELD) score. The scoring system was developed specifically for use in prediction of post-TIPS outcome.19 It provides a score based on a weighted mathematical formula including creatinine, bilirubin, and the international normalized ratio (INR): A 30-day mortality of 3.7% (1 in 27) is reported for patients with a MELD score of 1 to 10. Mortality increases to 60% (3 in 5) with a MELD score above 24.20 The Child-Pugh score has similar predictive effect on patient outcome after TIPS but may be slightly less accurate.21 The simplest prognostic measure is the serum bilirubin value alone. A bilirubin value over 3 mg/dL is associated with an increase in 30-day mortality after TIPS. Equipment required includes a TIPS set, a range of guidewires, angiographic catheters, angioplasty balloons, and stents (Table 112-1). Invasive pressure measuring equipment, appropriate patient monitoring facilities, and high-quality digital subtraction angiographic imaging are essential. Ultrasound imaging is also useful.
Transjugular Intrahepatic Portosystemic Shunts
Pathophysiology of Portal Hypertension
Indications
Variceal Hemorrhage
Ascites
Budd-Chiari Syndrome
Alternatives to Transjugular Intrahepatic Portosystemic Shunt
Contraindications
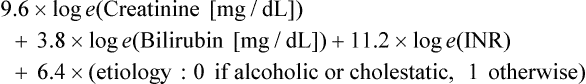
Equipment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine






