Treatment of the Failing Hemodialysis Graft
According to the 1997 U. S. Renal Data System report, Medicare paid for 73,000 hospital admissions and 123,000 outpatient procedures resulting from vascular access malfunction for dialysis in 1994. The estimated annual hospital-related cost for these procedures approached $1 billion per year.1 The unanticipated thrombosis of vascular access is the root cause of this high volume of hospitalizations and usually results in Radiologic or surgical intervention to restore access function.
Although significant improvements have been made in dialysis technology over the past decade, before 1995, no comprehensive effort had been made to standardize dialysis practice and to develop plans directed at improving the quality of life for dialysis patients. In 1995, the National Kidney Foundation-Dialysis Outcomes Quality Initiative (NKF-DOQI) was established. The NKF-DOQI was undertaken with the primary goal of improving patient outcomes and survival by providing literature-based recommendations for optimal clinical practice.
This chapter follows the specific guidelines set forth in the DOQI to evaluate a given access at risk. Based on these guidelines, we discuss the various percutaneous techniques that can be used to reduce graft thrombosis and thus limit underdialysis in this delicate patient population. Despite the use of any aggressive screening program, graft failure still will occur. Chapter 20 covers the topic of management of the failed graft with specific discussion of pharmacologic, pharmacomechanical, and mechanical thrombolysis.
 Initial Access Evaluation
Initial Access Evaluation
Wrist (radial-cephalic, i.e., Brescia-Cimino) and elbow (brachial-cephalic) primary fistulae are the preferred types of access. If a wrist or elbow fistula cannot be created, a dialysis anterovenous (AV) graft using synthetic materials [polytetrafluoroethylene (PTFE) or others] is the next choice. Synthetic dialysis access grafts have the advantages of providing a large surface area available for cannulation.2–5 The graft is technically easy to cannulate with multiple insertion sites available.2,6–9 The access can be created in a variety of shapes and configurations2–5,9–17 and are comparatively easy to repair surgically. The preferred graft site and configuration are the antecubital loop graft and the upper-arm curved graft. Grafts using smaller, more peripheral vessels may predispose the access site to more frequent thrombosis. This risk is weighed against the advantage of preserving more proximal sites for new grafts if this becomes necessary in the future.6,18–20
A variety of screening tests have been introduced to detect impending hemodialysis access failure. The specifics of such techniques are discussed elsewhere (see Chapter 23). Many of these examinations are performed in the dialysis unit or at the bedside. In dialysis AV grafts, thrombotic events result primarily from progressive venous outflow stenosis.16,18,21–27 Stenoses in the venous outflow tract are caused by intimal and fibromuscular hyperplasia (Fig. 19–1) and most typically occur at the vein graft anastomosis.16,18,21–23,25–27 As a stenosis progresses to hemodynamic significance (stenosis >50% diameter reduction/75 to 80% of cross-sectional area), it limits graft outflow with an increase in intraaccess pressure.28 A graft with blood flow of less than 600 mL per minute is at greater risk of thrombosis than a graft with a flow rate greater than 600 mL per minute.29–31 When access flow is measured repeatedly, the trend toward decreasing flows is of greater predictive value for access stenosis and thrombosis. Percutaneous or surgical intervention aimed at correcting the underlying stenosis can reduce dramatically the rate of graft thrombosis and access loss.16,18,21–23,27,32 When a test indicates the likely presence of a stenosis, venography or fistulography are used to confirm the lesion.
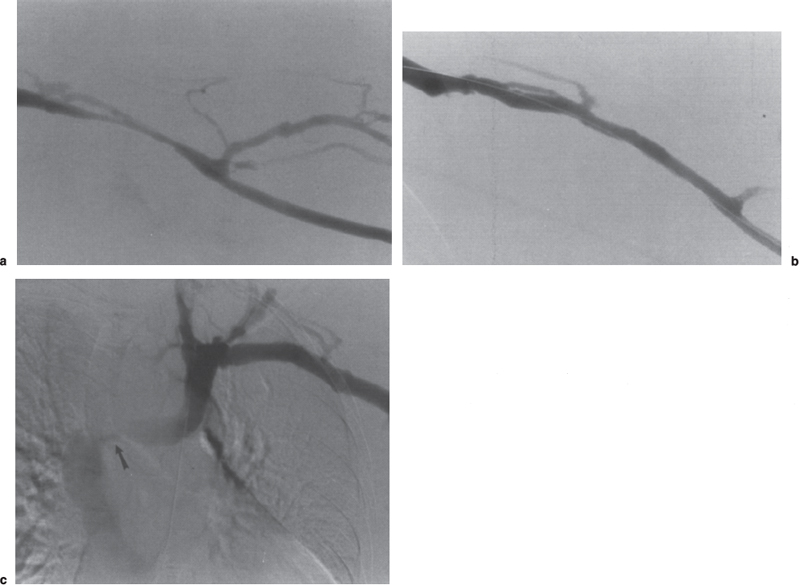
FIGURE 19–1. Typical venous outflow stenosis in polytetrafluoroethylene (PTFE) graft. (a) A hemodynamically significant anastomotic stenosis is detected on screening fistulography. The lesion is located in the native basilic vein just central to the venous anastomosis. (b) After successful percutaneous angioplasty, there is substantial improvement in the appearance of the stenosis. (c) The importance of a complete fistulogram is shown by the coexistence of a central venous stenosis involving the left innominate vein (arrow).
With respect to infection, tenderness and erythema along the graft as well graft fluctuance can indicate infection. These findings are important because graft infection is an absolute contraindication to percutaneous radiologic techniques. An untreated access infection may lead to bacteremia, sepsis, hemorrhage, and, if left untreated, possible death.6,7,10,36 In an infected access, the synthetic graft material acts as a foreign body. A newly placed graft that is not incorporated into the surrounding tissue requires total removal of the graft, even in the setting of a localized graft infection. Infection involving a mature AV graft requires both antibiotic therapy and surgical therapy to achieve a cure.6,37–39. To ensure resolution of extensive graft infection, treatment with antibiotics and surgical exploration and removal of any infected graft or graft segment are indicated.6,7,9,10,18,39
 Fistulography
Fistulography
Dialysis fistulography, because of its minimally invasive nature, cannot be thought of as a purely screening technique. If fistulography is performed immediately following dialysis with the needles still in place, the only risks involved to the patient are those associated with iodinated contrast material and further reduction in residual renal function, if any. Fistulography is an extremely low-risk procedure that remains the gold standard for evaluation of dialysis access grafts. A well-performed fistulogram is the foundation for all percutaneous interventions in hemodialysis access. The technique of fistulography has been well described.40–44 Careful attention to technique and detail in fistulography is paramount in preventing complications and missing pathology and in the satisfactory documentation of the outcome of percutaneous intervention. A complete fistulogram must evaluate the entire internal structure of the vascular access, including the inflow native artery and the outflow native vein to the level of the superior vena cava (Fig. 19–2). Occult central venous stenosis may occur in up to 50% of patients who have had prior catheters,45,46 and these stenoses may can account for repeated graft thrombosis even in the absence of gross arm swelling. Failure to evaluate the entire graft may result in the graft failing, despite an adequate technical result at the identified treatment site (Fig. 19–1C). Recent articles by Lumsden et al47–49 detected an average of 1.69 to 1.84 stenoses per screened patient. Safa et al50 also reported detecting multiple lesions in 35% (34 of 97) of cases in which an abnormality was detected on fistulography. This is most relevant to fistulography performed at the time of or immediately following surgical revision. This issue has been addressed in the DOQI guidelines,51 which recommend performing fistulography either during or immediately after all surgical procedures to evaluate the entire circulation and ensure that all potential areas of abnormality are detected and treated appropriately.
If needles are not already in place from dialysis, a number of different devices can be used to access the graft. The simplest and least expensive device is a 19-gauge butterfly needle or 18-gauge angiocath, both of which will admit a 0.035-inch guidewire should intervention become necessary. We prefer to perform our initial access using the Amplatz sheath needle (Becton-Dickinson, Franklin Lakes, NJ) or a single-wall 18-gauge needle can be used. Use of a 4F Micropuncture set (Cook, Bloomington, IN) can be helpful in accessing clotted or poorly palpable grafts but is usually unnecessary in routine access of patent grafts. The needle puncture site and orientation within the graft should be determined based on the initial preprocedure physical examination. Generally, because most stenoses occur at the venous anastomosis, the access needle should be directed antegrade toward the anastomosis. In anticipation of the need to reflux contrasts across the arterial anastomosis, however, positioning of the needle so as to allow for graft outflow compression may be helpful. Graft puncture can be facilitated by the use of a low-angle of entry of the needle so as not to puncture through the back wall of the graft. Puncture of the graft usually is detected by a characteristic “give.” When puncturing a thrombosed graft (for thrombolysis), blood return is not achieved, and the operator must often rely on this characteristic “give” to detect entry into the graft. In difficult situations, a limited contrast injection can be helpful in determining appropriate intragraft needle position. In this situation, care must be taken not to overinject the graft to avoid embolizing the arterial plug into the native artery.
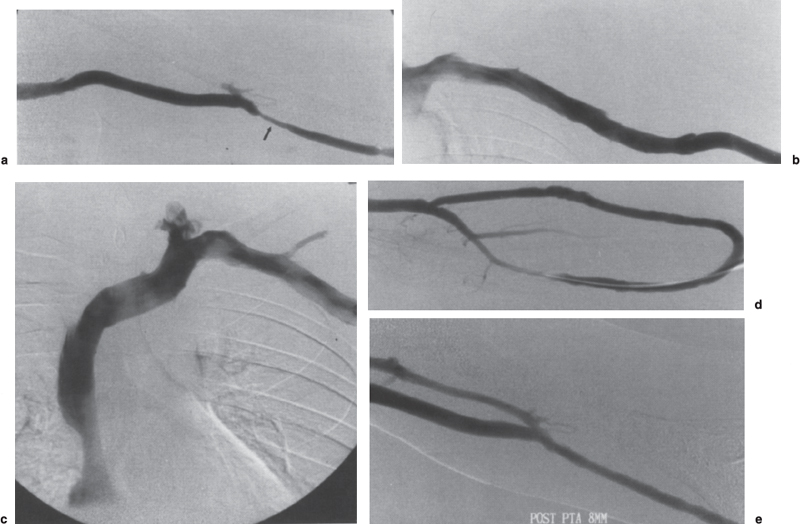
FIGURE 19–2. Complete dialysis fistulogram. (a) The forearm loop graft initially is imaged at the level of the venous anastomosis, which shows an anastomotic stenosis (arrow). (b,c) The basilica vein outflow and central venous structures are next studied to the level of the superior vena cava. (d) At the time of angioplasty of the outflow stenosis, the arterial limb is refluxed to visualize the arterial anastomosis. (e) Final assessment of the treatment site is performed showing an adequate technical result.
Like upper-extremity venography, fistulography should be performed with the arm in a slightly abducted position to avoid pseudostenosis of the axillary vein.52 The sequence of imaging should be from peripheral to central (Fig. 19–3). The initial site of evaluation should be based on physical examination, which usually points to the graft-native vein anastomosis, which is the most common site of access pathology. Rapid filming technique is helpful, particularly when multiple collaterals are present, which may act to obscure an underlying stenosis. Placing a blood-pressure cuff on the upper arm most easily allows for the evaluation of the arterial inflow. The cuff is inflated to a suprasystolic pressure followed by contrast injection. The outflow resistance allows visualization of the arterial anastomosis and the inflow native artery, often to the point of the blood pressure cuff centrally. It is important to attempt to image the anastomosis in profile whenever possible. Orthogonal views should be obtained at each level where pathology is suspected, which may entail turning the arm or hand while under fluoroscopy and while there is static of column of contrast to obtain the best angle. The blood-pressure cuff should be left up for the shortest possible time to avoid graft thrombosis. Patients should be advised that they might experience temporary discomfort from the arterial contrast during this part of the study. When evaluating upper-arm grafts, the use of a blood-pressure cuff is not possible; so manual compression of the graft outflow is often necessary to achieve reflux into the arterial circulation. Reflux also can be achieved by occluding the graft outflow using a balloon catheter. Once the arterial inflow has been evaluated, the remainder of the examination can be performed with the blood-pressure cuff deflated.
Angiographically, a hemodynamically significant stenosis is defined as a reduction of normal vessel diameter (graft or draining venous system of more than 50%). According to the DOQI guidelines, an angiographically significant stenosis must be accompanied by a hemodynamic, functional, or clinical abnormality before intervention is warranted.53 A stenosis should undergo percutaneous or surgical treatment if the stenosis is greater than 50% of the lumen diameter and is associated with (1) previous thrombosis in the access, (2) elevated venous dialysis pressure, (3) abnormal urea or other recirculation measurements, (4) abnormal physical findings (i.e., pulse in graft, swollen extremity), (5) unexplained decrease in measurement of dialysis dose, (6) decreasing access flow, or (7) unexplained reduction in Kt/V.
The rationale behind angiographic evaluation and treatment of the dialysis graft is that the presence of a venous stenosis increases the risk of thrombosis.30,32 Physiologically, a venous stenosis increases the resistance to blood flow, which in turn results in an increase of the intragraft venous pressure, decreased blood flow, and ultimately thrombosis.21,32 When examined angiographically, more than 90% of thrombosed grafts are associated with venous stenosis.54–59 In the absence of thrombosis, the presence of a graft outflow stenosis and the resulting derangement in flow reduce the efficiency of the dialysis treatment.27,60 The goal of therapeutic interventions for hemodynamically significant stenoses is to reduce the rate of graft thrombosis, graft loss, and prolongation of the average life of the access.21,23,32,61,62
Experience suggests, and the literature supports, the notion that long-term, cumulative graft patency is prolonged if the failing graft is treated before thrombosis occurs. Beathard59 showed that when a graft does fail, and thrombectomy is performed without addressing the presence of an underlying venous stenosis, there is a 90% or greater chance that the graft will rapidly rethrombose. A stenosis detected with the graft still patent is more responsive to intervention than one detected after thrombosis ensues. In a retrospective analysis, Katz and Kohl63 reported that when thrombosis was treated with thrombolysis and percutaneous transluminal angioplasty (PTA), the primary patency at 1 month was 50%. When the graft was still patent at the time of angioplasty, 50% of the grafts remained patent for 24 to 28 weeks. A 3-month patency of 78.9% for stenotic grafts treated before thrombus formation was reported by Beathard et al.22 In contrast, the primary patency rate for grafts averaged 40% at a similar interval when stenoses were corrected at the time of graft thrombolysis.22,34,64
In addition to an obvious high-grade hemodynamically significant stenosis, the presence of collateralization around a lesion should raise concern regarding the lesion significance. Thus, whenever collaterals are visualized on a fistulogram, the underlying cause should be sought (Fig. 19–4). If a cause is not detected readily, a catheter may be inserted through the access into the central veins and pullback pressure measurements performed to determine whether a pressure stepup is present at the site of the collaterals. Any significant pressure gradient (5 to 10 mm of mercury in central veins26 and 20 mm of mercury peripherally41) should be treated. The pullback technique of pressure measurements also can be useful in reducing contrast load. It should be noted that up to a 20 mm pressure gradient across the venous anastomosis is normal in a graft.65 In both fistulae and grafts, a smooth, gradual fall in pressure from the arterial side of the access into the central veins should occur. Any abrupt transition should be further examined and the cause treated. Occasionally, multiple vessels will fill during fistulography and give the appearance of collateral vessels in the absence of significant disease. A situation in which multiple venous systems may fill is when the venous anastomosis is placed astride or in close proximity to a venous bifurcation. In such cases, filling of two venous systems may not be abnormal. Also, if the venous anastomosis is created to the vena comitantes of the brachial artery, this network-like collection of veins surrounding the brachial artery frequently looks like collateral vessels and only rarely is straight-line flow achieved into the central circulation. Rather, this collateral-like network may show alternating areas of relative dilation and narrowing. Determination of significant stenoses in this type of vascular access can be difficult and may best be performed using the pullback pressure technique.
Another technique for assessing the significance of venous stenoses is the static pressure measurement technique reported by Sullivan et al.28,66 This group compared pressures determined at three locations within the graft to systemic pressures and found that pressures of greater than 33% of systemic at the venous and 50% at the arterial anastomoses correlated with graft failure. These numbers can be useful in determining the significance of a borderline stenosis as well as the adequacy of angioplasty or other intervention. In addition to use during fistulography, static pressure measurement eventually may be used as a screening technique if the inability of most dialysis machines to perform static pressure measurements is overcome.
In poorly functioning grafts, the most common abnormal finding is likely to be at the level of the vein-graft anastomosis or in the outflow vein central to the anastomosis (Fig. 19–5).25 Venous or vein-graft anastomotic lesions account for about 85% of stenoses,44 although significant variation is found in the reported incidence of such stenoses. In particular, there is a marked discrepancy between reports of surgical series, which report failure to detect a cause for access failure in 42 to 52% of cases67 and radiologic series in which an anatomic cause is detected in 90% or more of cases.57 Most authors attribute this discrepancy to failure to obtain intraoperative or postoperative fistulography combined with the improved quality and completeness of angiographic evaluation by radiologists.67–69 Arterial stenoses in grafts account for only about 5% or fewer of stenoses,44–70 although the reported incidence of arterial stenosis is higher in series in which clotted grafts were treated radiologically57,71 and surgically.10,26 This difference is most likely due to interpretation of residual thrombus at the arterial anastomosis as a fixed stenosis (see thrombolysis below). Intragraft stenoses constitute an additional 10% of all stenoses (Fig. 19–6).26,44 Most often, these are located at the two most frequent needle puncture sites because a buildup of intimal hyperplasia from repeated puncture72 or fibroblast ingrowth along the needle tracts. Such concentration of puncture sites leading to stenoses can be avoided by careful rotation of graft access sites in the dialysis unit. Another location for intragraft stenoses is at previous graft-graft anastomotic or graftotomy sites. Stenoses also may be present central to the venous anastomosis in the outflow arm veins or at the level of the central veins. Involvement of the outflow vein may occur at sites of vein wall trauma resulting from prior extremity venipuncture or in the setting of central vein stenosis from prior catheter insertion. For this reason, the DOQI guidelines include avoidance of peripheral venipuncture in patients at risk for or currently undergoing hemodialysis.73 Native vein stenosis also may develop at the site of valve leaflets, which can become thickened in response to the abnormally high venous flow associated with a functioning graft.26,44,52
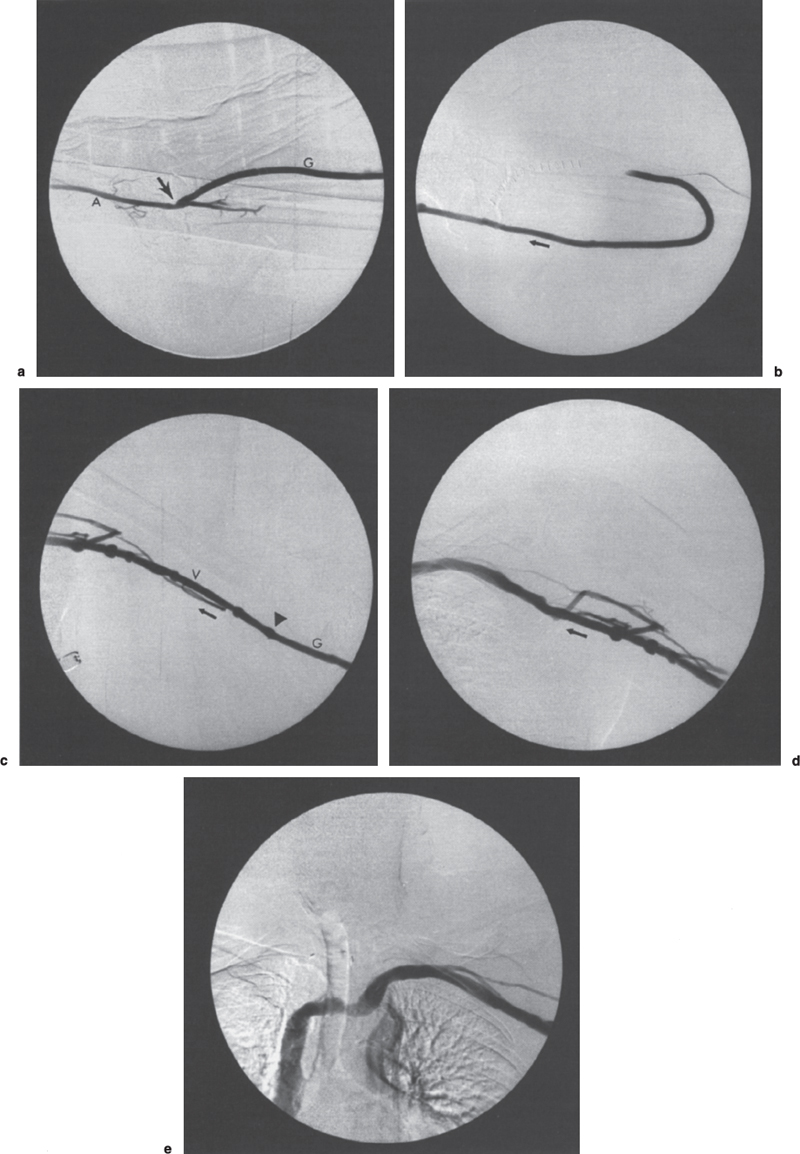
FIGURE 19–3. Normal dialysis fistulogram. This patient has a forearm loop polytetrafluoroethylene (PTFE) dialysis graft and underwent revision. This postoperative fistulogram is performed to detect any occult stenoses that might have been missed surgically. (a) Using a blood-pressure cuff on the upper arm, contrast is refluxed back to the arterial anastomosis, shown here in profile (arrow); A, brachial artery; G, graft. (b) Contrast injected with the blood-pressure cuff down shows the loop portion of the graft. Note the absence of stenosis, pseudoaneurysm, or residual thrombosis. Arrow indicates direction of flow. (c) Basilic vein in upper arm. Although a few small adjacent veins are opacified, which could be confused with collaterals, no underlying stenosis is present. These veins presumably opacified as a result of high flow. When in doubt, pullback blood pressure measurements are indicated to exclude significant stenosis (see text). Arrowhead indicates venous anastomosis. V, vein. Arrow indicates the direction of flow. (d) Axillary region. Again note the opacification of a few small parallel veins, which should not be confused with collaterals. No stenosis is identified. Arrow indicates direction of flow. (e) Central veins. Note the excellent opacification of axillary, subclavian, left innominate vein, and superior vena cava. No stenoses are identified. Note also the relative effacement of contrast column in the mid-left innominate vein, which most likely is due to mild extrinsic impression by the great arteries.
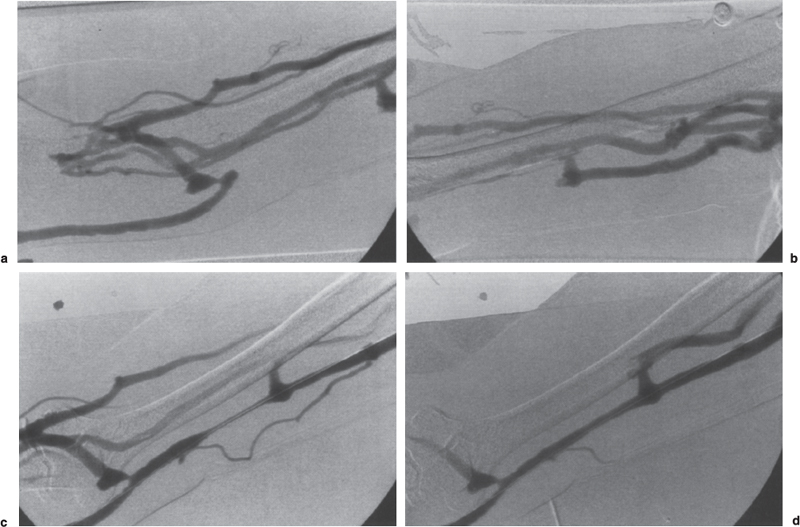
FIGURE 19–4. Collateral venous drainage around venous outflow occlusion. (a,b) Initial fistulogram shows multiple collaterals bypassing the occluded segment of the basilic vein outflow of the forearm loop-graft. The occluded segment was successfully traversed and balloon angioplasty performed. (c) After angioplasty, the fistulogram shows restoration of flow with residual narrowing within the treated vein segment. Note the decreased filling of the venous collaterals. (d) Following administration of nitroglycerin (100 μg/mL, total 2 mL), there is marked improvement in the appearance of the angioplasty site.
In addition to venous stenosis, other commonly detected abnormalities on fistulography include puncturesite pseudoaneurysm and graft degeneration. Aneurysmal dilation in PTFE grafts can be significant because it represents degeneration of the underlying graft. Graft degeneration (Fig. 19–7) is related to repeated puncture at identical sites with poor needle rotation. Often, this phenomenon is of little clinical significance in regard to adequate functioning of the graft. Pseudoaneurysm formation, especially the narrow neck type, can be associated with increased intragraft pressure and suggests the presence of a flow-limiting stenosis central to the site of pseudoaneurysm formation. Direct puncture of an area of graft degeneration should be avoided because of the risk of uncontrolled hemorrhage. Large pseudoaneurysms can prevent access to adjacent areas of the graft, thereby limiting potential puncture sites (Fig. 19–7). The DOQI guidelines recommend that pseudoaneurysms exceeding twice the diameter of the graft or those that are rapidly growing should be corrected surgically because of their increased risk of rupture.74 Pseudoaneurysms that are not resected may expand and rupture, resulting in significant blood loss. Pseudoaneurysm expansion that threatens the viability of the skin places the patient at risk of graft infection. In these cases, surgical correction is also indicated.
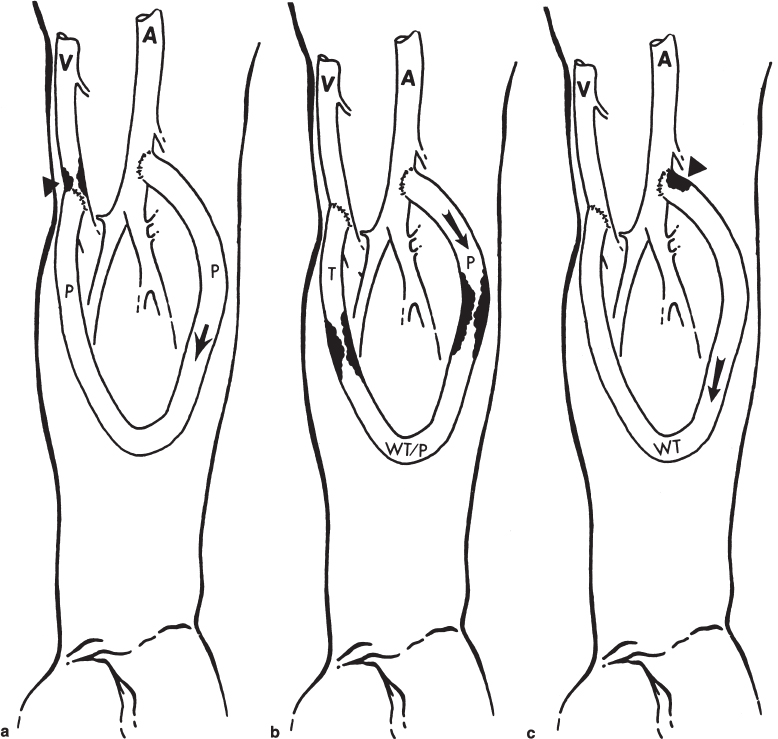
FIGURE 19–5. Typical patterns seen on fistulography of polytetrafluoroethylene (PTFE) bridge grafts. (a) Venous outflow stenosis. This is the most common location for stenosis, which consists of intimal hyperplasia and occurs just beyond the venous anastomosis (arrowhead). Arrow indicates direction of flow in this forearm loop graft. On physical examination, a pulse is found throughout the graft (p) with a thrill just beyond the venous stenosis. (b) Typical location of intragraft stenoses. When needle sites are poorly rotated, intimal hyperplasia may build up, with resultant intragraft stenosis. Arrow indicates direction of flow. On physical examination, a strong pulse is found on the arterial side of the first stenosis (p), a weak thrill/pulse between the two stenoses at the apex of the graft (wt/p), and a thrill beyond the second stenosis (t). (c) Arterial inflow lesion. These are the least common lesions found in hemodialysis access grafts. They may be due to either atherosclerotic stenoses in the inflow artery or more commonly to residual thrombus at the arterial anastomosis resulting from an incompletely removed arterial plug. The stenosis depicted here (arrowhead) is typical of residual arterial plug, but if it is left long enough, it may become organized into a fixed stenosis, which would require balloon angioplasty for treatment. On physical examination, this graft will have a weak thrill throughout (wt). The arrow indicates the direction of flow.
Steal syndrome refers to the reversal of flow in the efferent artery with resultant ischemia of the hand or digits.44 Steal can develop immediately following graft creation, or it may become clinically apparent along the course of the life of a graft. Although the diagnosis of steal can be made clinically or by ultrasound, fistulography may detect a treatable cause, such as stenosis of the afferent or efferent artery. It is important to recognize that bidirectional flow, or even reversal of flow in the efferent artery, is extremely common and that simple detection of this physiology does not warrant the diagnosis in the absence of clinical symptoms.44,75 In addition, other causes of ischemia, such as arterial emboli from prior thrombectomies or thrombolysis, may be detected.
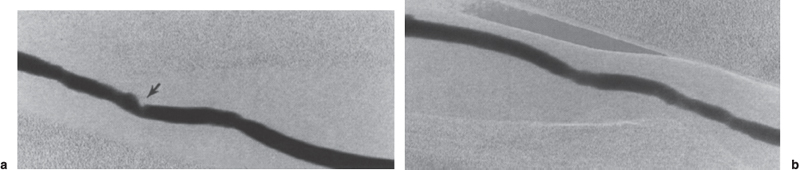
FIGURE 19–6. Intragraft stenosis, (a) Intragraft stenosis within the venous limb of a polytetrafluoroethylene (PTFE) graft (arrow). Intragraft stenoses most often result from poor rotation of needle access sites during hemodialysis sessions or may be due to residual adherent thrombus remaining following graft thrombolysis. (b) Appearance of the venous limb following balloon angioplasty to 7 mm.
Dialysis access thrombosis usually is detected clinically. Grafts that no longer have a palpable pulse or thrill are clearly thrombosed and do not need further evaluation to make the diagnosis of access failure. With respect to thrombosed grafts, we found some utility in performing arm venography via the thrombosed graft by placing a catheter into the graft and threading it up into the venous circulation to perform fistulography. This procedure usually is done in anticipation of percutaneous thrombolysis, but in patients whose venous outflow looks too severely diseased to accomplish thrombolysis, the information gained from this venography is extremely useful to the access surgeon in planning revascularization.58 In addition, occult central venous stenoses can be detected preoperatively, which may prompt a decision to find another access site for the patient. Rarely, a fistulogram may be performed in a failing graft during the process of thrombosis in which the graft is only partially occluded. In this case, rapid institution of angioplasty of the offending stenosis usually results in restoration of flow without need for thrombolysis per se.
In summary, careful attention to detail when performing the fistulogram and the judicious use of adjuncts such as pressure measurements will help to avoid diagnostic errors. Only after the successful completion of a thorough study can attention be turned to the treatment of the abnormality at hand.

FIGURE 19–7. Pseudoaneurysm formation. Direct puncture of an area of graft degeneration should be avoided because of the risk of uncontrolled hemorrhage. In this case, a large pseudoaneurysm of the venous limb was accessed with the dialysis needle (arrowhead) through which the fistulogram has been performed. The arterial limb needle also has been placed into an area of graft degeneration (arrow). Large pseudoaneurysms can prevent access to adjacent areas of the graft and thereby limit potential puncture sites.
 Pathogenesis and Treatment of Graft Stenosis
Pathogenesis and Treatment of Graft Stenosis
As can be determined from the preceding discussion, venous outflow stenosis accounts for most failures of vascular access, particularly synthetic grafts. It is this venous stenosis and its response or lack of thereof to a given treatment modality that ultimately determine the longterm results of therapy, be it by percutaneous or surgical means. The natural history of most graft failures is one of gradual progression of stenosis at the graft-vein anastomosis. Such stenoses are due to intimal hyperplasia with progressive increases in graft pressure and reduction in flow. If these abnormalities are not detected using screening modalities, the graft progresses to thrombosis. If flow is restored by simple thrombectomy without treatment of the outflow stenosis, rapid rethrombosis will occur in many patients.18,76 The typical stenosis encountered is a focal, concentric lesion. The vein wall at this location contains an exuberant num ber of smooth-muscle cells associated with increased amounts of extracellular matrix.77 The stimulus for intimal response is the hemodynamic stress and turbulence experienced locally in and around the anastomotic region. Energy is dissipated into the perivascular tissues surrounding the vessel. The result is a chronic stimulus promoting smooth-muscle cell recruitment and proliferation. The extent of response is directly related to the degree of turbulence present.78 Of the various graft configurations, loop-graft configurations have a theoretic advantage. In this type of graft configuration, energy is dissipated around the loop with less energy in the form of turbulence dissipated at the venous anastomosis. Safa et al50 also has shown improved primary patency following angioplasty when comparing loop with straight configuration grafts. Although the nature of the hypertrophied intima is relatively well characterized, efforts to prevent its development have yet progress to the point where widespread clinical application is warranted. The most recent method to be applied toward the prevention of restenosis has been radiation therapy. Experience gained in coronary application suggests the potential for clinical benefit. Use in dialysis access has been limited to testing within animal models and small case series with overall poor result.79 Obviously, if intimal hyperplasia could be prevented, so could most graft failures. If and until such methods are developed, however, therapies aimed at treating the resulting stenosis will remain the mainstay of percutaneous and surgical management.
The traditional approach to venous outflow stenosis was and continues in many institutions to be surgical revision.16–18,76,80,81 Surgical treatment of the failing graft usually involves patch angioplasty or the placement of an interposition graft or both. This type of management results in acceptable patency rates but offers the distinct disadvantage of depleting a segment of vein with each subsequent revision. Consequently, after several revisions, there is no further usable vein, and access must be sought elsewhere in the same extremity or in another extremity. Percutaneous management of venous stenosis dates back to the early 1980s. Dialysis-related venous angioplasty is now one of the most frequently performed procedures by interventional radiology. The DOQI guidelines make no determination as to the preferred method of treating the failing access but recommend that each dialysis center determine which procedure (i.e., angioplasty or surgical revision) is best for the patient based on the expertise at that center.82
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree