Treatment of the Thrombosed Hemodialysis Graft
The Dialysis Outcomes Quality Initiative (DOQI) guidelines recommend that the target cumulative patency rate for an arteriovenous (AV) graft should be 70%, 60% and 50% at 1, 2 and 3 years, respectively.1 Despite efforts to detect and treat venous stenosis before it results in thrombosis, thrombosis can occur without warning, even with a dedicated screening program in place. In addition to the typical venous outflow stenosis, graft thrombosis can result from poor cardiac output, vascular (arterial) insufficiency, hypotension during dialysis or other hypotensive events, faulty compression technique, compression of the graft during sleep, and the presence of a hypercoagulable state. Whatever the underlying cause, thrombosis of hemodialysis access is a major contributor to patient morbidity and hemodialysis access maintenance costs.
The main goals in the treatment of the failed access include (1) availability of services so that dialysis can be resumed in an expedient fashion; (2) safe and effective restoration of flow in the graft; and (3) an ability to provide alternate means of dialysis, such as central venous dialysis catheters, if the thrombolysis procedure does fail. It is important to recognize that significant time and effort need to be dedicated on the part of the interventional radiology service to achieve these goals.
The timing of graft repair relative to the time of failure is controversial. The current trend is to treat graft salvage as an urgent procedure. This approach has been taken in an effort to spare the patient the morbidity and mortality associated with missed hemodialysis or temporary catheterization. The DOQI guidelines state that treatment of the failed access should be performed soon after graft thrombosis is detected to avoid the need for temporary access placement.2 With this commitment to timely care, the patient with a failed access can be quite disruptive to the regular schedule of an interventional service. Occasionally, restoring flow to the graft cannot be performed in a timely fashion, necessitating temporary catheterization. Once a temporary catheter is in place, the question becomes whether diminishing returns are associated with delaying treatment of the clotted graft. This question was partially addressed in a paper showing that for polytetrafluorethylene (PTFE) grafts, successful salvage could be achieved as long as 3 weeks from the time of failure.3 In this longitudinal study, no significant difference in survival was found for any period of delay if successful salvage was achieved compared with patients who received a new access. Thus, in this setting, an attempt can be made to declot a graft that has been thrombosed for up to several weeks, and we have successfully declotted grafts that have been thrombosed for months. Nonetheless, we make every effort to treat the graft within the time frame suggested by the DOQI. Practically, this means that patients presenting with thrombosed grafts on the first or second dialysis weekday shift will be treated either the same day (if NPO for conscious sedation) or the next day. Third-shift patients are treated the next day. If the patient presents on Friday third shift or the weekend, the procedure is performed Monday morning.
Surgical and percutaneous treatment options are available for clotted dialysis grafts and fistulae. Surgical interventions include simple surgical thrombectomy, surgical thrombectomy with patch angioplasty, and thrambectomy with graft revision. Percutaneous therapy for the failed hemodialysis access includes pharmacologic (infusion), pharmacomechanical, and mechanical thrombolysis. Regardless of the percutaneous technique chosen, the patient must be carefully evaluated, including his or her access history, before graft salvage is attempted. The interventional radiologist must work in close cooperation with both the nephrologist and the access surgeon to ensure that the appropriate procedure is performed. In the setting of recurrent graft failure, lacking a contraindication to surgery, the patient may be better served by surgical thrombectomy and graft revision. We use two percutaneous declotting failures within a 1-month period as justification for pursuing surgical repair with access revision. In addition, a patient with a history of severe pulmonary artery hypertension, intrinsic lung disease, or documented right-to-left shunt is considered at risk of central embolization from thrombolysis and may be better served by surgical thrombectomy. Patients with severe allergic histories to iodinated contrast material need to be premedicated appropriately before the procedure. In the case of an acutely ill patient (e.g., one with severe hyperkalemia or volume overload), emergent hemodialysis may be required and is best performed following placement of a temporary dialysis catheter. More definitive treatment of the occluded graft then can be performed electively.
In the setting of a recently failed graft following surgical creation or revision, pharmacologic or pharmacomechanical (but not mechanical) thrombolysis may be contraindicated because of the increased risk of a hemorrhagic complication. A significant and probably unrecognized contraindication to percutaneous treatment is graft infection. The appropriate management for an infected graft is surgical resection,4 in part because of the risk of septic embolization following percutaneous intervention. Patient mortality from central septic embolization following mechanical thrombolysis of an occluded dialysis graft was reported.5 Ayus and Sheikh-Hamad6 evaluated the incidence of occult infection of thrombosed, nonfunctioning grafts. The authors found purulent material in all of 20 patients studied who presented with fever or with fever and signs of sepsis but no clinical evidence of graft infection. What is more alarming was the finding of purulent material in 13 of 21 of completely asymptomatic patients presenting with clotted grafts. For this reason, a careful history and physical examination are important as is the routine administration of antibiotics [e.g., cefazolin 1 g intravenously (i.v.)] before performing graft thrombolysis.
 Pharmacomechanical Thrombolysis of the Failed Graft
Pharmacomechanical Thrombolysis of the Failed Graft
Early efforts at percutaneous thrombolytic therapy in clotted dialysis-access grafts and fistulae involved the systemic infusion of thrombolytic agents and often required inpatient hospitalization for overnight infusion. Initially, the agent of choice was streptokinase, which was changed to urokinase because of the high complication rate, especially bleeding complications at the infusion site or locally from prior graft access sites.7–12 In addition to bleeding complications, streptokinase therapy cannot be repeated because of antibody formation; this problem renders streptokinase therapy less useful for treatment of the failed graft, which tends to require treatment more frequently than every 6 months. Most pharmacologic thrombolytic regimens for the failed hemodialysis access provide for the use of urokinase (Abbokinase, Abbott Laboratories, North Chicago, IL). Early experience with urokinase involved either direct injection into the graft with a needle13,14 or with an angled catheter.11 Similar to streptokinase, early use of urokinase involved prolonged infusion performed over a period of hours or overnight. Despite moderate success in achieving thrombolysis with urokinase infusion, significant bleeding complications were common,11,15 and the expense of keeping the patient in the hospital overnight, usually in the intensive care unit, was prohibitive.
These shortcomings limited the widespread application of pharmacologic techniques for treating the failed access and might have led to its demise if not for the development of pharmacomechanical thrombolysis. In its embryonic stage, the technique for pharmacomechanical thrombolysis, as described by Davis et al16 used concentrated urokinase deposited throughout the length of the clotted access, followed by clot maceration with angled catheters placed in a crisscross fashion. The initial results using the crossed-catheter technique16 demonstrated adequate technical success with lysis in 90% of the 41 cases, with an average procedure time of 86 minutes (± 57 minutes). The cumulative patency rate was reported to be 62% at 6 months. This approach gained moderate acceptance but was hampered by the significant drawback of being relatively time consuming.
Refinement of the technique continued, and it evolved into what is now known as pulse-spray pharmacomechanical thrombolysis (PSPMT), which, as reported by Valji et al17 (the same group as the initial description by Davis et al16) involves the percutaneous placement of two multi-sidehole infusion catheters in opposite directions in the graft (crossed-catheter technique), followed by injection of small aliquots of highly concentrated urokinase (25,000 U of urokinase per milliliter) using a tuberculin syringe. A total of 150,000 U of urokinase are administered over 15 to 20 minutes. The patient is given 5000 to 7000 U of i.v. heparin from the onset of urokinase therapy. In their initial report, this approach required an average of 299,000 ± 125,000 U of urokinase and required a mean of 46 ± 21 minutes to complete. This process compared favorably with their prior technique of thrombus lacing and subsequent clot maceration. Using this approach, these authors reported an initial success rate of 93%, with a 1-year primary patency of 26% and 1-year accumulative patency of 51%.17
Although the authors report high technical success, the concern for graft rethrombosis prompted investigation into the use of combined urokinase and heparin in the pulse-spray solution. Rethrombosis is due primarily to platelet activation, increased thrombin activity, and release of plasminogen-activator inhibitors. The main action of heparin in this regard is its direct binding with and subsequent inactivation of antithrombin III. This action can “tip the balance“ in favor of more effective thrombolysis without concomitant rethrombosis. Although antithrombin III is scarce in a clot, the addition of heparin improved overall thrombolysis.18 A comparative study performed with and without heparin in the pulse-spray mixture demonstrated a decrease in mean urokinase dose by an average of 315,000 U with an average time savings of 42 minutes in the heparin group.19 Subsequent reports by the group at University of California San Diego included intrathrombus injection of heparin along with urokinase.20,21 The UCSD technique has now been refined to the point where a single 250,000 U vial of urokinase is used along with both intravenous and intrathrombotic heparin (total 8000 U).22 The urokinase and heparin admixture is delivered by pulse-spray technique over 10 to 15 minutes. Residual thrombus then is macerated by use of an angioplasty balloon. In the setting of significant residual thrombus, additional urokinase can be used to avoid its central embolization. The relatively lysis-resistant, arterial plug is cleared by the passage of an occlusion balloon beyond the arterial anastomosis, where it is then inflated and pulled back toward the graft. The plug then is disrupted by maceration using the same angioplasty balloon, after which any underlying stenosis is treated.
Other authors, using slight modifications in the described technique,23 achieved similar results using the pulse-spray technique. Brunner et al24 described a technique, which they called ultrarapid urokinase, which used an infusion technique through multi-sidehole catheters to achieve 1-hour lysis in 79% of grafts. Berger et al25 showed the pulse-spray thrombolysis can be used after recent surgery, which was previously thought to be a contraindication to pharmacological thrombolytic therapy.25 Twelve procedures performed within 30 days after surgical thrombectomy yielded a clinical success rate of 75%, with a mean primary patency of 94 days.
A recent variation on the technique of pharmacologic thrombolysis was described by Cynamon et al.26 Using the “lyse and wait“ technique, the author initially evaluated the patient and blindly punctured the graft using a 22-gauge angiocatheter. Using this technique, a mixture of 250,000 U of urokinase and 5000 U of heparin in 10 mL is injected into the graft while compressing the arterial and venous ends. The patient is brought into the angiography suite when 30-120 minutes following injection and the graft is studied angiographically. The resistant arterial plug then is typically treated, as is the outflow stenosis. In the 18 reported cases, the initial angiogram following the “lyse and wait“ showed no or minimal residual intragraft thrombus. In all cases, an arterial plug was mechanically mobilized and an outflow stenosis treated. The clinical success, as defined by at least one successful hemodialysis session, was 94%. Although the author advocated this technique for decreasing the procedure time, no analysis of this variable was performed. In this limited series, no clinically significant arterial or pulmonary emboli was reported. Several potential problems exist with this technique, including (1) attempting thrombolysis before venous outflow is evaluated; (2) blind injection of the urokinase mixture; (3) the volume of mixture injected, with the inherent risk of embolization, especially arterial; and (4) the administration of urokinase to an unmonitored patient. Until these issues are addressed, we cannot recommend routine use of this technique.
 Mechanical Thrombolysis of the Failed Graft
Mechanical Thrombolysis of the Failed Graft
The basic tenets of mechanical thrombolysis are (1) crossed-catheter access; (2) the use of a mechanical device or technique to macerate or pulverize the clot; (3) embolization or aspiration of the resulting particulate; and (4) treatment of the underlying cause of graft thrombosis, usually a venous outflow stenosis. Mechanical thrombolysis of hemodialysis grafts was developed to address a number of problems associated with chemical thrombolysis: (1) bleeding complications from the multiple puncture sites in these grafts, (2) prolonged procedure times, (3) the cost and risk of the thrombolytic agent, and (4) residual clot in the graft. In addition, the specific issue of safety has been raised, particularly in regard to the risk of infectious complications from the use of Abbokinase (urokinase, Abbott Laboratories, North Chicago, IL). A warning letter from the U. S. Food and Drug Administration (FDA) to health care providers27 stated that “the likelihood that cases of infectious diseases caused by Abbokinase, if any, would have been recognized as such and reported to FDA is probably low. Therefore, the actual risk to patients of developing an infectious disease as a result of using Abbokinase is unknown.” As of this writing, urokinase is no longer available and it is unclear whether it will ever be reintroduced.
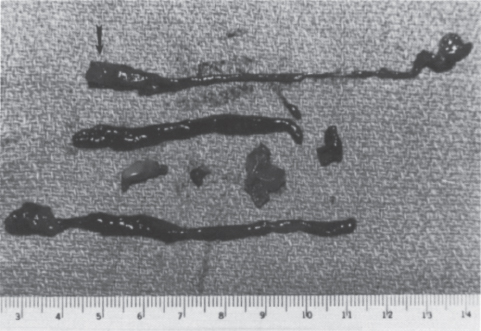
FIGURE 20–1. Specimen from the surgical thrombectomy of a failed polytetrafluoroethylene (PTFE) graft. The vast majority of the thrombus within the graft is soft, red thrombus or socalled stasis thrombus. The arterial plug (arrow) is shown grossly as firm, whitish clot recovered from the arterial edge of the graft. It has a concave surface as a result of compaction from arterial pulsation.

FIGURE 20–2. Microscopic section through the arterial plug. The cross-sectional view of the arterial plug demonstrates well-defined, concentric layers oriented toward the arterial surface (arrowhead) of the graft. The dense layering and compaction account for its resistance to pharmacologic thrombolysis.
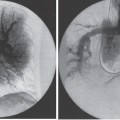
The most significant impediments to restoration of flow in a dialysis graft are the underlying outflow stenosis, usually at the venous anastomosis, and the arterial plug. To understand mechanical thrombolysis and its importance relative to chemical techniques, one must understand the composition of clot in a thrombosed graft (Fig. 20–1). In one series, the volume of a solid clot in an average-length (42 cm) thrombosed PTFE graft of the forearm in loop configuration is approximately 3 cc, and never more than 5 cc.28 The clot is predominantly soft red thrombus, which fragments or lyses relatively easily. Unlike most of the graft contents, the clot at the level of the arterial anastomosis is firm, Compacted, and mature white thrombus consisting of alternating layers of red cells and platelets or fibrin (Fig. 20–2). On gross examination, the “arterial plug“ is a cylindrical piece of white clot with a concave surface facing the artery, leading many surgeons to describe it as the “bullet.“ Surgeons consider removal of the arterial plug the sine qua non of successful thrombectomy. Failure to remove this plug results in decreased patency after thrombectomy.29 The arterial plug is well documented to be extremely resistant to chemical thrombolysis, initially thought to be due to the platelet richness of the clot,17–21 but more recently thought to be due to the dense compaction and layering within the plug.30 The urokinase used in PSPMT does not lyse the arterial plug, nor does it improve the venous outflow stenosis. Because these are the two most important determinants of success of the thrombolysis procedure, it is not surprising that the results of mechanical thrombolysis have been equivalent to techniques using thrombolytic agents. Again, it must be emphasized that the long-term result of any thrombolytic technique is probably dictated by the result of treatment of the underlying stenosis, not by the individual thrombolytic technique.
Pulse-spray Saline Technique
The concept of the primary role of urokinase in PSPMT first was challenged by Beathard.31 In a prospective, randomized study, pharmacomechanical (PM) thrombolysis (n = 48) was compared with pulse-spray mechanical (M) thrombolysis (n = 55) using heparinized saline alone. The PM group received 250,000 U of urokinase and 4,000 U of heparin in 10 mL delivered in 0.6-mL pulses ever four minutes. The M group received 2000 U of heparin in 10-mL pulses and pulsed as described. The goal of treatment was not complete thrombolysis but the return of graft flow. In both groups, balloon maceration of residual thrombus was performed. The clinical success rates for the M and PM groups were 92.8% and 93.8%, respectively. The 30-and 90-day patency was 65 vs 72% and 37% vs 46% for the M and PM groups, respectively. No instance of peripheral embolization or symptomatic pulmonary embolus occurred in either treatment group. The only statistically significant difference was that M group took less time to complete (mean, 48 minutes) than the PM group (mean 58 minutes). This well-designed study demonstrated that clot maceration with pulse saline was safe and effective in restoring function to thrombosed access grafts without the need or cost of a thrombolytic agent. Beathard et al32 since published their long-term results of mechanical thrombolysis with pulse-spray heparinized saline in 1176 patients. The clinical success rate was 96% of treated patients, with a decrease in the mean procedure time to 33 minutes. The primary patency rates were 74%, 52%, 39%, and 17% at 1, 3, 6, and 12 months, respectively. No difference in patency was found for patients undergoing their first thrombolysis procedure compared with those undergoing second, third, or fourth procedures.
Percutaneous Mechanical Balloon Declotting
Mechanical declotting (Fig. 20–3) without urokinase makes use of a balloon catheter to push the clot from the venous limb into the central circulation.33 Following a second retrograde access in the venous limb, the “arterial plug“ is dislodged by using a Fogarty catheter, which is used to clear the plug, usually centrally. In the original report,33 the mean lysis time was 62 minutes and the mean procedure time 116 minutes. The technical success rate (achieving lysis) was 94%, with an initial clinical success rate (patency at 7 days) of 59%. The projected 30-day patency was 56%. Using this technique, no symptomatic pulmonary embolus was reported. The procedure is contraindicated in patients who have significant pulmonary disease or right-to-left shunts. The long-term effect of repeated procedures is not known, and the author recommends further study. This basic technique was compared with pulse-spray thrombolysis using urokinase by the group at the University of Pennsylvania.34 In this study, technical and clinical success rates were similar and the mean primary patency rate was identical for both treatment groups. A significant difference between study groups was found in mean procedure times (3.5 hours versus 2.2 hours for the pharmacomechanical and mechanical thrombolysis groups, respectively). The single major complication (arterial embolus) occurred in the pharmacomechanical thrombolysis group and was treated with continued urokinase infusion. In a follow-up to the original series, the same group reported outcomes from a total of 86 mechanical declotting procedures.5 In this series, a single death from sepsis occurred. The death presumably was related to an occult graft infection, with septic emboli to the lung caused by the mechanical declotting procedure. This patient had no record of receiving preprocedure antibiotics.
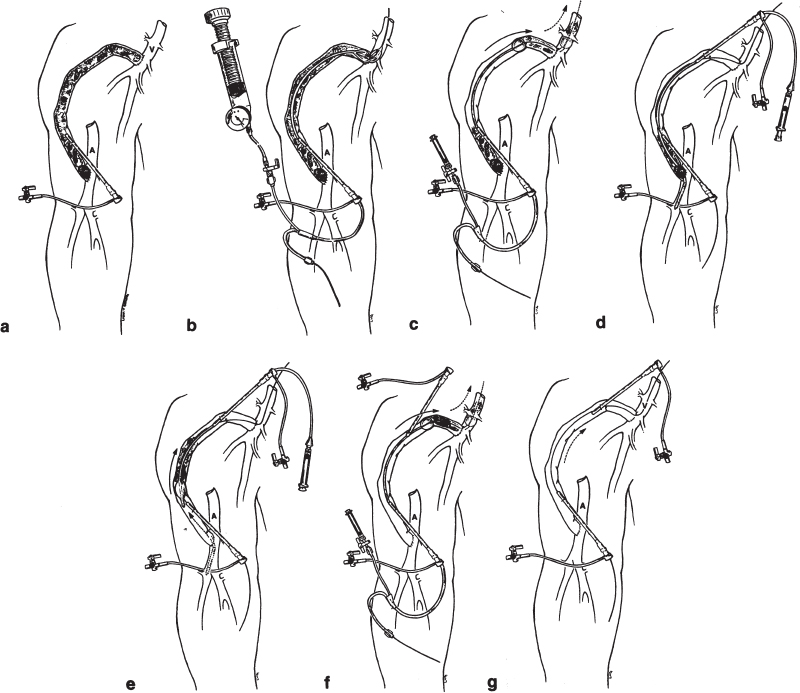
FIGURE 20–3. Balloon mechanical declotting technique. Schematic representation of clotted right upper-arm polytetrafluoroethylene (PTFE) dialysis graft. (a) Initially, a 5F sheath is placed directed toward the venous anastomosis using a micropuncture set for initial access. (b) A 6-or 7-mm angioplasty balloon is placed in the vicinity of the venous anastomosis and dilated using an insufflator with a pressure gauge. This predilation allows clot to pass easily through the venous anastomotic region, which usually has a stenosis. (c) After administration of a minimum of 3000 U of intravenous heparin, a .025-inch guidewire is negotiated through the venous anastomosis and over this guidewire a 5F wedge balloon is used to push the clot into the central venous circulation. (d) A second 5F sheath is placed, again using the micropuncture set, directed toward the arterial anastomosis. It is important that the two sheaths not overlap to avoid compromising flow in the graft once it is restored. Through this sheath, a 5F Fogarty catheter is placed and negotiated through the arterial anastomosis. (e) The Fogarty is inflated and withdrawn into the graft, dislodging the arterial plug as well as any residual thrombus. (f) If necessary, the wedge balloon is again used through the venous sheath to push any residual clots centrally. (g) At completion of the procedure, there should be a thrill in the graft. Complete fistulography is performed, and any underlying stenoses are treated.
In a variation of the standard intragraft approach, a central vein access was used to perform thrombolysis of an occluded graft mechanically.35 From a central venous access position, an occlusion balloon is advanced retrograde across the arterial anastomosis. The balloon then is withdrawn through the graft and back into the central veins. Using this technique, the authors achieved technical success in 25 of 31 patients (81%). The median procedure time was 115 minutes (range, 16-278 minutes). Prolonged procedure times were encountered in patients with an access stenosis that required additional graft punctures to traverse the obstruction. The primary patency of grafts that were declotted successfully was 33%, 23%, and 13% at 3, 6, and 12 months, respectively. The advantages of this approach are the ability to thrombectomize the graft using a single antegrade motion, the elimination of crossed catheters in the graft, and the ability to limit radiation exposure to the operator.
Mechanical thrombectomy using thromboaspiration has been reported with relatively good results in conjunction with fibrinolysis.36 Sharafuddin et al37 described a balloon-assisted aspiration technique for graft thrombectomy. Using this technique in an in vitro flow circuit, the authors were able to aspirate effectively 98% of the thrombus by volume, with only 2% embolized into the venous outflow. No thrombus was embolized into the arterial inflow. We have used such a technique in about 200 patients with technical success exceeding 90% (Fig. 20–4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree