Treatment Planning II: Patient Data, Corrections, and Setup
Basic depth dose data and isodose curves are usually measured in a cubic water phantom having dimensions much larger than the field sizes used clinically. Phantom irradiations for this purpose are carried out under standard conditions, for example, beams incident normally on the flat surface at specified distances. The patient’s body, however, is neither homogeneous nor flat in surface contour. Thus, the dose distribution in a patient may differ significantly from the standard distribution. This chapter discusses several aspects of treatment planning, including acquisition of patient data, correction for contour curvature, and tissue inhomogeneities and patient positioning.
12.1. Acquisition of Patient Data
Accurate patient dosimetry is only possible when sufficiently accurate patient data are available. Such data include body contour, outline, and density of relevant internal structures, location, and extent of the target volume. Acquisition of these data is necessary whether the dosimetric calculations are performed manually or with a computer. However, this important aspect of treatment planning is often executed poorly. For example, in a busy department there may be an inordinate amount of pressure to begin the patient’s treatment without adequate dosimetric planning. In other cases, lack of sufficient physics support and/or equipment is the cause of this problem. In such a case, it must be realized that the final accuracy of the treatment plan is strongly dependent on the availability of the patient data and that great effort is needed to improve its quality.
A. Body Contours
Acquisition of body contours and internal structures is best accomplished by imaging (computed tomography [CT] and magnetic resonance imaging, etc.). The scans are performed specifically for treatment-planning purposes, with the patient positioned the same way as for actual treatment. In 3-D treatment planning (Chapter 19) these data are all image based and are acquired as part of the treatment-planning process. However, for cases in which 3-D treatment planning is not considered necessary or if body contours are obtained manually for verification of the image-based contours, mechanical or electromechanical methods are used for contouring.
A number of devices have been made to obtain patient contours. Some of these are commercially available, while others can be fabricated in the department machine shop. The most common and the simplest of the devices is a solder wire or a lead wire embedded in plastic. Because the wire may not faithfully retain the contour dimensions when transferring it from the patient to the paper, one must independently measure anteroposterior and/or lateral diameters of the contour with a caliper.
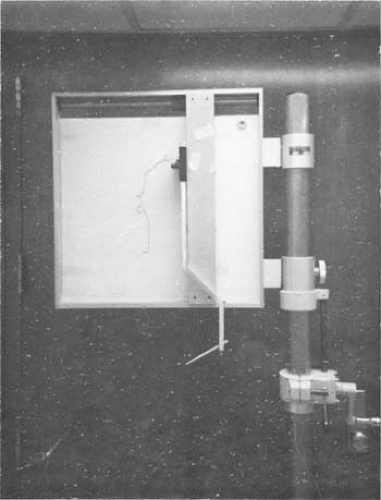
Another kind of simple device (1) consists of an array of rods, the tips of which are made to touch the patient’s skin and then placed on a sheet of paper for contour drawing. Perhaps the most accurate of the mechanical devices is a pantograph-type apparatus (Fig. 12.1) in which a rod can be moved laterally as well as up and down. When the rod is moved over the patient contour, its motion is followed by a pen that records the outline on paper.
Clarke (2) has described an electromechanical device in which motion of the rod over the patient contour is read by a sensing device and transferred to an X-Y recorder. Such a device can be used for digitizing the patient contour for direct input to the treatment-planning computer. Optical (3) and ultrasonic (4) methods have also been devised to obtain the contour information.
Although any of the above methods can be used with sufficient accuracy if carefully used, some important points must be considered in regard to manual contour making:
The patient contour must be obtained with the patient in the same position as used in the actual treatment. For this reason, probably the best place for obtaining the contour information is with the patient properly positioned on the treatment simulator couch.
A line representing the tabletop must be indicated in the contour so that this horizontal line can be used as a reference for beam angles.
Important bony landmarks as well as beam entry points, if available, must be indicated on the contour.
Checks of body contour are recommended during the treatment course if the contour is expected to change due to a reduction of tumor volume or a change in patient weight.
If body thickness varies significantly within the treatment field, contours should be determined in more than one plane.
B. Internal Structures
Localization of internal structures for treatment planning should provide quantitative information in regard to the size and location of critical organs or inhomogeneities. Although qualitative information can be obtained from diagnostic radiographs or atlases of cross-sectional anatomy, they cannot be used directly for precise localization of organs relative to the external contour. In order for the contour and the internal structure data to be realistic for a given patient, the localization must be obtained under conditions similar to those of the actual treatment position and on a couch similar to the treatment couch.
The following devices are used for the localization of internal structures and the target volume. A brief discussion regarding their operation and function will be presented.
B.1. Computed Tomography
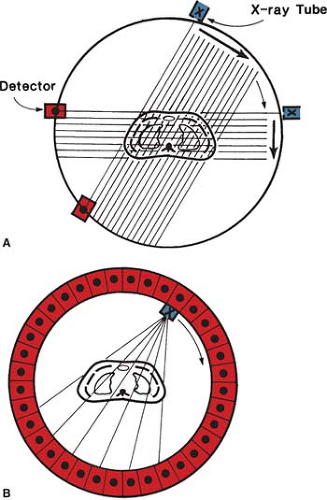
The main disadvantage of conventional transverse tomography is the presence of blurred images resulting from structures outside the plane of interest. In CT, the x-rays used for reconstructing the image enter only the layer under examination, so that unwanted planes are completely omitted. Basically, a narrow beam of x-rays scans across a patient in synchrony with a radiation detector on the opposite side of the patient. If a sufficient number of transmission measurements are taken at different orientations of the x-ray source and detector (Fig. 12.2A), the distribution of attenuation coefficients within the layer may be determined. By assigning different levels to different attenuation
coefficients, an image can be reconstructed that represents various structures with different attenuation properties. Such a representation of attenuation coefficients constitutes a CT image.
coefficients, an image can be reconstructed that represents various structures with different attenuation properties. Such a representation of attenuation coefficients constitutes a CT image.
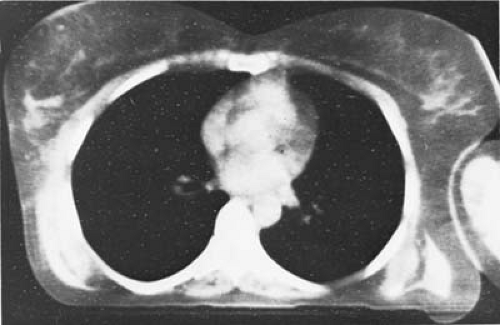
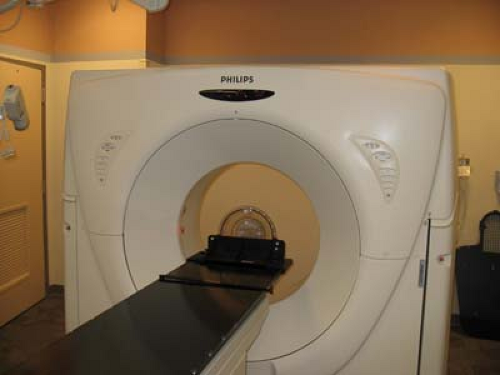
Since CT scanning was introduced about 30 years ago, there has been a rapid development in both the software and hardware. Most of the improvements in hardware had to do with the scanner motion and the multiplicity of detectors to decrease the scan time. Figure 12.2B illustrates a modern scanner in which the x-ray tube rotates within a circular array of 1,000 or more detectors. With such scanners, scan times as fast as 1 second or less are achievable. Figure 12.3 shows a typical CT image.
Spiral or helical CT scanners were introduced in the early 1990s in which the x-ray tube rotates continuously as the patient is slowly translated through the CT aperture. Helical CTs are faster and provide better visualization of anatomy and target volumes.
The reconstruction of an image by CT is a mathematical process of considerable complexity, generally performed by a computer. For a review of various mathematical approaches for image reconstruction, the reader is referred to a paper by Brooks and Di Chiro (5). The reconstruction algorithm generates what is known as CT numbers, which are related to attenuation coefficients. The CT numbers range from –1,000 for air to +1,000 for bone, with that for water set at 0. The CT numbers normalized in this manner are called Hounsfield numbers (H):
where μ is the linear attenuation coefficient. Thus, a Hounsfield unit represents a change of 0.1% in the attenuation coefficient of water.
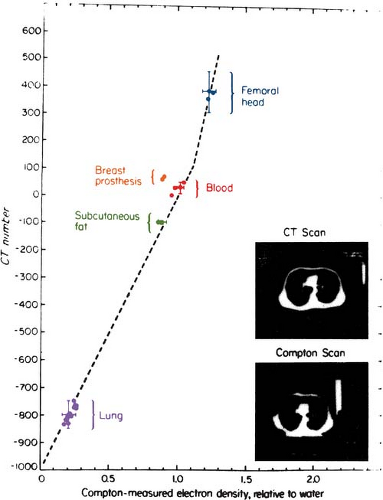
Because the CT numbers bear a linear relationship with the attenuation coefficients, it is possible to infer electron density (electrons cm-3) as shown in Figure 12.4. Although CT numbers can be correlated with electron density, the relationship is not linear in the entire range of tissue densities. The nonlinearity is caused by the change in atomic number of tissues, which affects the proportion of beam attenuation by Compton versus photoelectric interactions. Figure 12.5 shows a relationship that is linear between lung and soft tissue but nonlinear between soft tissue and bone.
Atomic number information can also be obtained if attenuation coefficients are measured at two different x-ray energies (6). It is possible to transform the attenuation coefficients measured by CT at diagnostic energies to therapeutic energies (7). However, for low-atomic-number materials such as fat, air, lung, and muscle, this transformation is not necessary for the purpose of calculating dose distributions and inhomogeneity corrections (7).
Application of CT in radiation therapy treatment planning has been the subject of many papers (7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22). The CT information is useful in two aspects of treatment planning: (a) delineation of target volume and the surrounding structures in relation to the external contour and (b) providing quantitative data (in the form of CT numbers) for tissue heterogeneity corrections. From a practical point of view, the first aspect is more important than the second. Accurate delineation of surface contour, internal structures, and target volume is not only crucial for optimizing a treatment technique, but also necessary for accurate calculation of dose distribution. Even the tissue heterogeneity
corrections for megavoltage photon beams can be made with acceptable accuracy by using the CT cross-sectional image to determine the extent of the inhomogeneity and employing published values of electron density. To quote Sontag et al. (10), “The most severe errors in computing the dose distribution are caused by inaccurate delineation of the geometric outlines of tissue inhomogeneities. Less severe errors in the dose calculation are caused by using an inaccurate relative electron density for the inhomogeneity, provided the outline is accurate.” Similar observations were also made by Geise and McCullough (9).
corrections for megavoltage photon beams can be made with acceptable accuracy by using the CT cross-sectional image to determine the extent of the inhomogeneity and employing published values of electron density. To quote Sontag et al. (10), “The most severe errors in computing the dose distribution are caused by inaccurate delineation of the geometric outlines of tissue inhomogeneities. Less severe errors in the dose calculation are caused by using an inaccurate relative electron density for the inhomogeneity, provided the outline is accurate.” Similar observations were also made by Geise and McCullough (9).
From these investigations, it seems that more sophisticated inhomogeneity correction algorithms such as those using “pixel-by-pixel” CT data produce only small improvements in dose accuracy compared with the traditional methods using equivalent depth, provided the extent of the inhomogeneity is accurately known. However, greater precision in the inhomogeneity outline and electron density may be required in regions where severe dose gradients exist in the direction of the beam. For example, the electron density information may be critical for some cases of electron beam therapy and, for high-energy photons, in regions where electronic equilibrium is not established. In such instances, methods using pixel-by-pixel correction methods may be required (14,23). Also, in the case of lung correction, dose calculation algorithms based on electron transport such as convolution/superposition or Monte Carlo are necessary to provide accuracy of better than 5% (discussed in Chapter 19).
There are several commercially available computer treatment-planning systems that allow display and use of CT images for treatment planning. Once the CT image has been produced, either
the data can be transferred to the treatment-planning computer directly or the outlines of the contour and internal structures can be traced by hand and then entered into the computer. In direct systems, the CT scan is displayed in grayscale mode on the computer monitor of the treatment- planning system and relevant structures can be outlined on the basis of CT number distribution. After the treatment plan is finalized, it can be superimposed on the CT image for visual display.
the data can be transferred to the treatment-planning computer directly or the outlines of the contour and internal structures can be traced by hand and then entered into the computer. In direct systems, the CT scan is displayed in grayscale mode on the computer monitor of the treatment- planning system and relevant structures can be outlined on the basis of CT number distribution. After the treatment plan is finalized, it can be superimposed on the CT image for visual display.
The use of CT scans in treatment planning is now an established procedure. Comparative studies of treatment planning with and without CT have demonstrated significantly improved accuracy of target delineation, field shaping, and normal tissue exclusion from the field when treatments are designed with the aid of CT scans. A review of CT applications in radiotherapy is presented in a book edited by Ling et al. (24). More current applications are discussed in part III of this book.
Although external contour and internal structures are well delineated by CT, their use in treatment planning requires that they be localized accurately with respect to the treatment geometry. Diagnostic CT scans obtained typically on a curved tabletop with patient position different from that to be used in treatment have limited usefulness in designing technique and dose distribution. Special treatment-planning CT scans are required with full attention to patient positioning and other details affecting treatment parameters.
Some of the common considerations in obtaining treatment-planning CT scans are the following: (a) a flat tabletop should be used; usually a flat wooden board can be designed to provide a removable insert for the diagnostic CT couch; (b) a large-diameter CT aperture (e.g., ≥70 cm) can be used to accommodate unusual arm positions and other body configurations encountered in radiation therapy; (c) care should be taken to use patient-positioning or immobilization devices that do not cause image artifacts; (d) patient positioning, leveling, and immobilization should be done in accordance with the expected treatment technique or simulation if done before CT; (e) external contour landmarks can be delineated using radiopaque markers such as plastic catheters; (f) sufficiently magnified images for digitization can be obtained if radiographs on film are to be used for drawing target and other structures; and (g) image scale should be accurate both in the X and Y directions.
B.2. Three-dimensional Treatment Planning
Additional considerations go into CT scanning for 3-D treatment planning. Because the 3-D anatomy is derived from individual transverse scans (which are imaged in 2-D), the interslice distance must be sufficiently small to reconstruct the image in three dimensions. Depending on the tumor site or the extent of contemplated treatment volume, contiguous scans are taken with slice thickness ranging from 2 to 10 mm. The total number of slices may range from 30 to 80 mm. This requires fast scan capability to avoid patient movement or discomfort.
Delineation of target and critical organs on each of the scans is necessary for the 3-D reconstruction of these structures. This is an extremely time-consuming procedure, which has been a deterrent to the adoption of 3-D treatment planning on a routine basis. Efforts have been directed toward making this process less cumbersome such as automatic contouring, pattern recognition, and other computer manipulations. However, the basic problem remains that target delineation is inherently a manual process. Although radiographically visible tumor boundaries can be recognized by appropriate computer software, the extent of target volume depends on grade, stage, and patterns of tumor spread to the surrounding structures. Clinical judgment is required in defining the target volume. Obviously, a computer cannot replace the radiation oncologist! At least, not yet.
Besides the time-consuming process of target localization, 3-D computation of dose distribution and display requires much more powerful computers in terms of speed and storage capacity than the conventional treatment-planning systems. However, with the phenomenal growth of computer technology, this is not perceived to be a significant barrier to the adoption of routine 3-D planning.
Whereas 3-D planning for every patient may not be necessary, it has already been found to be useful and practical for most tumors or tumor sites (head and neck, lung, prostrate). Treatment of well-localized small lesions (e.g., <4 cm in diameter) in the brain or close to critical structures by stereotactic radiosurgery has greatly benefited from 3-D planning. In this procedure, the target volume is usually based on the extent of radiographically visible tumor (with contrast), thus obviating the need for manual target delineation on each CT slice. The 3-D display of dose distribution to assess coverage of the target volume confined to a relatively small number of slices is both useful and practical. Similarly, brachytherapy is amenable to 3-D planning because of the limited number of slices involving the target.
The next best thing to full-fledged 3-D planning (e.g., 3-D computation and display) is to do 2-D planning, using a limited number of CT scans, selected to obtain a reasonably adequate perspective of the dose distribution in three dimensions. For example, targets and other critical structures may be drawn on 5 to 10 CT cuts, spanning the volume of interest. This can be done either by drawing targets and structures on the CT films or directly on the computer screen by using a cursor or a light pen. Treatment-planning software is available whereby margins around the target volume can be
specified to set the field boundaries. After optimizing field margins, beam angles, and other plan parameters relative to the central CT cut, the dose distributions can be viewed in other slices either individually or simultaneously by serial display on the screen. Beam’s eye view (BEV) display in which the plan is viewed from the vantage point of the radiation source (in a plane perpendicular to the central axis) is useful in providing the same perspective as a simulator or port film. In addition, a BEV outline of the field can be obtained to aid in the drawing of custom blocks on the simulator film. More discussion on CT-based treatment planning is provided in Chapter 19.
specified to set the field boundaries. After optimizing field margins, beam angles, and other plan parameters relative to the central CT cut, the dose distributions can be viewed in other slices either individually or simultaneously by serial display on the screen. Beam’s eye view (BEV) display in which the plan is viewed from the vantage point of the radiation source (in a plane perpendicular to the central axis) is useful in providing the same perspective as a simulator or port film. In addition, a BEV outline of the field can be obtained to aid in the drawing of custom blocks on the simulator film. More discussion on CT-based treatment planning is provided in Chapter 19.
B.3. Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) has developed, in parallel to CT, into a powerful imaging modality. Like CT, it provides anatomic images in multiple planes. Whereas CT provides basically transverse axial images (which can be further processed to reconstruct images in other planes or in three dimensions), MRI can be used to scan directly in axial, sagittal, coronal, or oblique planes. This makes it possible to obtain optimal views to enhance diagnostic interpretation or target delineation for radiotherapy. Other advantages over CT include not involving the use of ionizing radiation, higher contrast, and better imaging of soft tissue tumors. Some disadvantages compared with CT include lower spatial resolution; inability to image bone or calcifications; longer scan acquisition time, thereby increasing the possibility of motion artifacts; technical difficulties due to small hole of the magnet and magnetic interference with metallic objects; and current unavailability of many approved MRI contrast agents. The previous cursory comparison between CT and MRI shows that the two types of imaging are complementary.
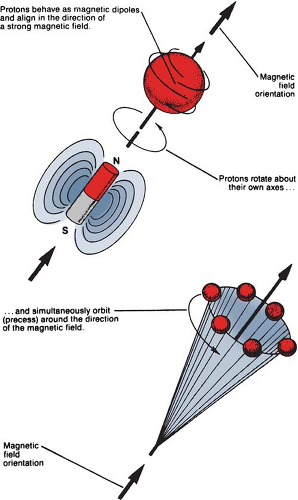
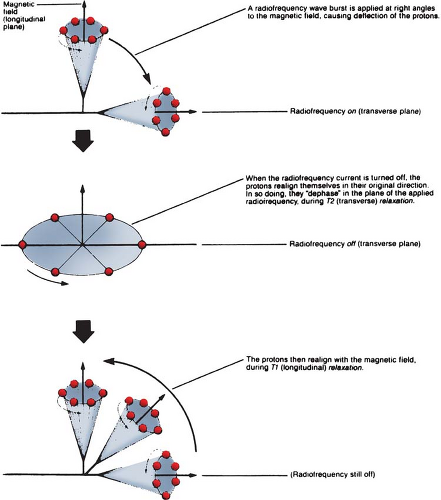
Basic physics of MRI involves a phenomenon known as nuclear magnetic resonance (NMR). It is a resonance transition between nuclear spin states of certain atomic nuclei when subjected to a radiofrequency (RF) signal of a specific frequency in the presence of an external magnetic field. The nuclei that participate in this phenomenon are the ones that intrinsically possess spinning motion (i.e., have angular momentum). These rotating charges act as tiny magnets with associated magnetic dipole moment, a property that gives a measure of how quickly the magnet will align itself along an external magnetic field. Because of the spinning motion or the magnetic dipole moment, nuclei align their spin axes along the external magnetic field (H) as well as orbit or precess around it (Fig. 12.5). The frequency of precession is called the Larmor frequency. A second alternating field is generated by applying an alternating voltage (at the Larmor frequency) to an RF coil. This field is applied perpendicular to H and rotates around H at the Larmor frequency. This causes the nuclei to precess around the new field in the transverse direction. When the RF signal is turned off, the nuclei return to their original alignment around H. This transition is called relaxation. It induces a signal in the receiving RF coil (tuned to the Larmor frequency), which constitutes the NMR signal.
The turning off of the transverse RF field causes nuclei to relax in the transverse direction (T2 relaxation) as well as to return to the original longitudinal direction of the magnetic field (T1 relaxation). This is schematically illustrated in Figure 12.6. The relaxation times, T1 and T2, are actually time constants (like the decay constant in radioactive decay) for the exponential function that governs the two transitions.
The signal source in MRI can be any nucleus with nonzero spin or angular momentum. However, certain nuclei give larger signal than the others. Hydrogen nuclei (protons), because of their high intrinsic sensitivity and high concentration in tissues, produce signals of sufficient strength for imaging. Work with other possible candidates, 31P, 23Na, 19F, 13C, and 2H, is continuing. Currently, routine MRI is based exclusively on proton density and proton relaxation characteristics of different tissues.
Localization of protons in a 3-D space is achieved by applying magnetic field gradients produced by gradient RF coils in three orthogonal planes. This changes the precession frequency of protons spatially, because the MR frequency is linearly proportional to field strength. Thus, by the appropriate interplay of the external magnetic field and the RF field gradients, proton distribution can be localized. A body slice is imaged by applying field gradient along the axis of the slice and selecting a frequency range for a readout. The strength of the field gradient determines the thickness of the slice (the greater the gradient, the thinner the slice). Localization within the slice is accomplished by phase encoding (using back-to-front Y gradient) and frequency encoding (using transverse X gradient). In the process, the computer stores phase (angle of precession of the proton at a particular time) and frequency information and reconstructs the image by mathematical manipulation of the data.
Most MR imaging uses a spin echo technique in which a 180-degree RF pulse is applied after the initial 90-degree pulse, and the resulting signal is received at a time that is equal to twice the interval between the two pulses. This time is called the echo time (TE). The time between each 90-degree pulse in an imaging sequence is called the repetition time (TR). By adjusting TR and TE, image contrast can be affected. For example, a long TR and short TE produces a proton (spin) density-weighted image, a short TR and a short TE produces a T1-weighted image, and a long TR and a long
TE produces a T2-weighted image. Thus, differences in proton density, T1, and T2 between different tissues can be enhanced by a manipulation of TE and TR in the spin echo technique.
TE produces a T2-weighted image. Thus, differences in proton density, T1, and T2 between different tissues can be enhanced by a manipulation of TE and TR in the spin echo technique.
Figure 12.7 shows examples of MR images obtained in the axial, sagittal, and coronal planes. By convention, a strong MR signal is displayed as white and a weak signal is displayed as dark on the cathode ray tube or film.
B.4. Ultrasound
Ultrasonic imaging for delineating patient contours and internal structure is becoming widely recognized as an important tool in radiation therapy. Tomographic views provide cross-sectional information that is always helpful for treatment planning. Although in most cases the image quality or clinical reliability is not as good as that of the CT, ultrasonic procedure does not involve ionizing radiation, is less expensive, and in some cases, yields data of comparable usefulness.
Ultrasound can provide useful information in localizing many malignancy-prone structures in the lower pelvis, retroperitoneum, upper abdomen, breast, and chest wall (25). A detailed review of these techniques in the context of radiation therapy planning has been presented by Carson et al. (25,26).
An ultrasound (or ultrasonic) wave is a sound wave having a frequency greater than 20,000 cycles per second or hertz (Hz). At this frequency, the sound is inaudible to the human ear. Ultrasound waves of frequencies 1 to 20 MHz are used in diagnostic radiology.
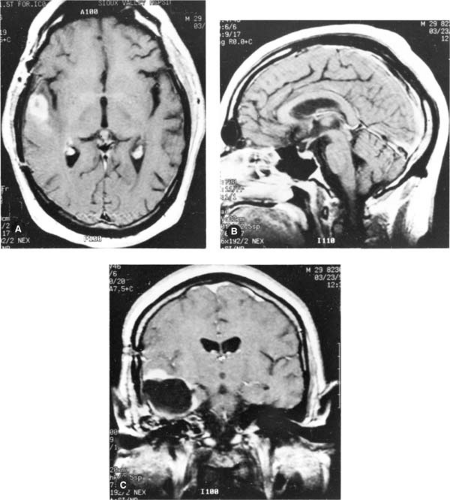 Figure 12.7. Examples of magnetic resonance images of the head. A: Transverse plane. B: Sagittal plane. C: Coronal plane. |
Ultrasound may be used to produce images either by means of transmission or reflection. However, in most clinical applications, use is made of ultrasonic waves reflected from different tissue interfaces. These reflections or echoes are caused by variations in acoustic impedance of materials on opposite sides of the interfaces. The acoustic impedance (Z) of a medium is defined as the product of the density of the medium and the velocity of ultrasound in the medium. The larger the difference in Z between the two media, the greater is the fraction of ultrasound energy reflected at the interface. For example, strong reflections of ultrasound occur at the air–tissue, tissue–bone, and chest wall–lung interfaces due to high impedance mismatch. However, because lung contains millions of air–tissue interfaces, strong reflections at the numerous interfaces prevent its use in lung imaging.
Attenuation of the ultrasound by the medium also plays an important role in ultrasound imaging. This attenuation takes place as the energy is removed from the beam by absorption, scattering, and reflection. The energy remaining in the beam decreases approximately exponentially with the depth of penetration into the medium, allowing attenuation in different media to be characterized by attenuation coefficients. As the attenuation coefficient of ultrasound is very high for bone compared with soft tissue, together with the large reflection coefficient of a tissue–bone interface, it is difficult to visualize structures lying beyond bone. On the other hand, water, blood, fat, and muscle are very good transmitters of ultrasound energy.
Ultrasonic waves are generated as well as detected by an ultrasonic probe or transducer. A transducer is a device that converts one form of energy into another. An ultrasonic transducer converts electrical energy into ultrasound energy, and vice versa. This is accomplished by a process known as
the piezoelectric effect. This effect is exhibited by certain crystals in which a variation of an electric field across the crystal causes it to oscillate mechanically, thus generating acoustic waves. Conversely, pressure variations across a piezoelectric material (in response to an incident ultrasound wave) result in a varying electrical potential across opposite surfaces of the crystal.
the piezoelectric effect. This effect is exhibited by certain crystals in which a variation of an electric field across the crystal causes it to oscillate mechanically, thus generating acoustic waves. Conversely, pressure variations across a piezoelectric material (in response to an incident ultrasound wave) result in a varying electrical potential across opposite surfaces of the crystal.
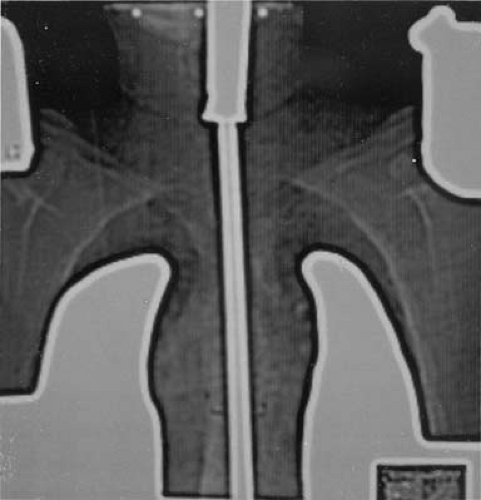
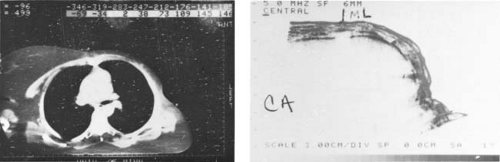 Figure 12.8. Ultrasonic tomogram showing chest wall thickness (right) compared with the computed tomography image (left). |
Although the piezoelectric effect is exhibited by a number of naturally occurring crystals, most common crystals used clinically are made artificially such as barium titanate, lead zirconium titanate, and lead metaniobate. The piezoelectric effect produced by these materials is mediated by their electric dipole moment, the magnitude of which can be varied by addition of suitable impurities.
As the ultrasound wave reflected from tissue interfaces is received by the transducer, voltage pulses are produced that are processed and displayed on the cathode ray tube (CRT), usually in one of three display modes: A (amplitude) mode, B (brightness) mode, and M (motion) mode. A mode consists of displaying the signal amplitude on the ordinate and time on the abscissa. The time, in this case, is related to distance or tissue depth, given the speed of sound in the medium. In the B mode, a signal from a point in the medium is displayed by an echo dot on the CRT. The (x, y) position of the dot on the CRT indicates the location of the reflecting point at the interface and its proportional brightness reveals the amplitude of the echo. By scanning across the patient, the B-mode viewer sees an apparent cross section through the patient. Such cross-sectional images are called ultrasonic tomograms.
In the M mode of presentation, the ultrasound images display the motion of internal structures of the patient’s anatomy. The most frequent application of M-mode scanning is echocardiography. In radiotherapy, the cross-sectional information used for treatment planning is exclusively derived from the B-scan images (Fig. 12.8). The use of ultrasound in brachytherapy (e.g., ultrasound-guided prostate implants) is discussed in Chapter 23.
12.2. Treatment Simulation
A. Radiographic Simulator
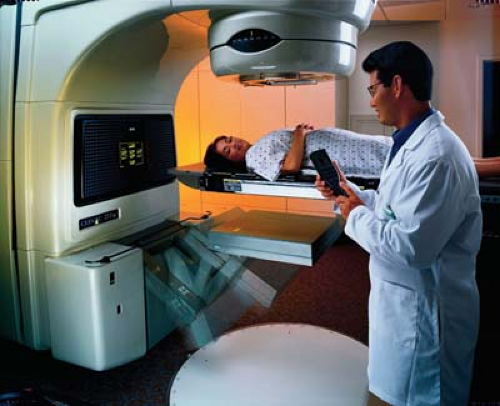
A treatment simulator (Fig. 12.9) is an apparatus that uses a diagnostic x-ray tube but duplicates a radiation treatment unit in terms of its geometric, mechanical, and optical properties. The main function of a simulator is to display the treatment fields so that the target volume may be accurately encompassed without delivering excessive irradiation to surrounding normal tissues. By radiographic visualization of internal organs, correct positioning of fields and shielding blocks can be obtained in relation to external landmarks. Most commercially available simulators have fluoroscopic capability by dynamic visualization before a hard copy is obtained in terms of the simulator radiography.
Specifications of a treatment simulator must closely match those of the treatment unit. Several authors (27,28,29,30,31) have discussed these aspects. For a comprehensive discussion, the reader is referred to the paper by McCullough and Earl (31).
The need for simulators arises from four facts: (a) geometric relationship between the radiation beam and the external and internal anatomy of the patient cannot be duplicated by an ordinary diagnostic x-ray unit; (b) although field localization can be achieved directly with a therapy machine by taking a port film, the radiographic quality is poor because of very high beam energy, and for cobalt-60, a large source size as well; (c) field localization is a time-consuming process that, if carried out in the treatment room, could engage a therapy machine for a prohibitive length of time; and (d) unforeseen problems with a patient setup or treatment technique can be solved during simulation, thus conserving time within the treatment room.
Although the practical use of simulators varies widely from institution to institution, the simulator room has assumed the role of a treatment-planning room. Besides localizing treatment volume and setting up fields, other necessary data can also be obtained at the time of simulation. Because the simulator table is supposed to be similar to the treatment table, various patient measurements such as contours and thicknesses, including those related to compensator or bolus design, can be obtained under appropriate setup conditions. Fabrication of immobilization devices and testing of individualized shielding blocks can also be accomplished with a simulator. To facilitate such measurements, simulators are equipped with accessories such as laser lights, contour maker, and shadow tray.
Some simulators have a tomography attachment in which the image from the image intensifier is analyzed and reconstructed using either analog1 or digital2 processing. Because of the poor image quality, this technology cannot compete with CT-based virtual simulation.
Modern simulators combine the capabilities of radiographic simulation, planning, and verification in one system. These systems provide commonality in hardware and software of the treatment machine including 2-D and 3-D imaging, accessory mounts, treatment couch, and multileaf collimator (MLC). One such system is Acuity (Varian Medical Systems, Palo Alto, CA) (Fig. 12.9B).
B. CT Simulator
An exciting development in the area of simulation is that of converting a CT scanner into a simulator. CT simulation uses a CT scanner to localize the treatment fields on the basis of the patient’s CT scans. A computer program, specifically written for simulation, automatically positions the patient couch and the laser cross-hairs to define the scans and the treatment fields. The software (as part of CT scanner or a stand-alone treatment-planning system) provides outlining of external contours, target volumes and critical structures, interactive portal displays and placement, review of multiple treatment plans, and a display of isodose distribution. This process is known as virtual simulation.
The nomenclature of virtual simulation arises out of the fact that both the patient and the treatment machine are virtual—the patient is represented by CT images and the treatment machine is modeled by its beam geometry and expected dose distribution. The simulation film in this case is a reconstructed image called the DRR (digitally reconstructed radiograph), which has the
appearance of a standard 2-D simulation radiograph but is actually generated from CT scan data by mapping average CT values computed along ray lines drawn from a “virtual source” of radiation to the location of a “virtual film.” DRR is essentially a calculated (i.e., computer-generated) port film that serves as a simulation film. The quality of the anatomic image is not as good as the simulation radiograph but it contains additional useful information such as the outlined target area, critical structures, and beam aperture defined by blocks or MLC. Figure 12.10 shows an anterior field DRR (A) and a posterior oblique field DRR (B). A DRR can substitute for a simulator radiograph by itself but it is always preferable to obtain final verification by comparing it with a radiographic simulation film.
appearance of a standard 2-D simulation radiograph but is actually generated from CT scan data by mapping average CT values computed along ray lines drawn from a “virtual source” of radiation to the location of a “virtual film.” DRR is essentially a calculated (i.e., computer-generated) port film that serves as a simulation film. The quality of the anatomic image is not as good as the simulation radiograph but it contains additional useful information such as the outlined target area, critical structures, and beam aperture defined by blocks or MLC. Figure 12.10 shows an anterior field DRR (A) and a posterior oblique field DRR (B). A DRR can substitute for a simulator radiograph by itself but it is always preferable to obtain final verification by comparing it with a radiographic simulation film.
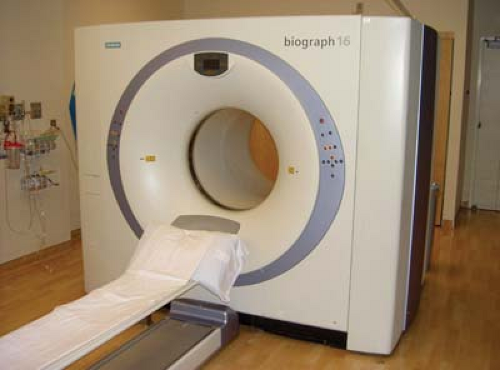
A dedicated radiation therapy CT scanner with simulation accessories (e.g., flat table, laser lights for positioning, immobilization and image registration devices, and appropriate software for virtual simulation) is called a CT simulator. Many types of such units are commercially available. Figure 12.11 shows one example.
C. PET/CT
Positron emission tomography (PET) provides functional images that can differentiate between malignant tumors and the surrounding normal tissues. This capability can be combined with the anatomic information provided by a CT scanner to complement each other. The idea of combining both of these modalities into a single system for simulation has led to the development of PET/CT.
A PET/CT unit consists of PET and CT scanners combined together with a common patient couch (Fig. 12.12). Because the patient position on the couch is kept constant for both of the scanning procedures, it is possible to fuse the information from the two scanners. The composite simulation image contains more information than is possible by a CT simulator alone.
The physics of PET involves positron-electron annihilation into photons. For example, a radiolabeled compound such as fluorodeoxyglucose (FDG) incorporates 18F as the positron-emitting
isotope. FDG is an analog of glucose that accumulates in metabolically active cells. Because tumor cells are generally more active metabolically than the normal cells, an increased uptake of FDG is positively correlated with the presence of tumor cells and their metabolic activity. When the positron is emitted by 18F, it annihilates a nearby electron, with the emission of two 0.511-MeV photons in opposite directions. These photons are detected by ring detectors placed in a circular gantry surrounding the patient. From the detection of these photons, a computer software (e.g., filtered back-projection algorithm) reconstructs the site of the annihilation events and the intervening anatomy. The site of increased FDG accumulation, with the surrounding anatomy, is thereby imaged with a resolution of approximately 4 mm.
isotope. FDG is an analog of glucose that accumulates in metabolically active cells. Because tumor cells are generally more active metabolically than the normal cells, an increased uptake of FDG is positively correlated with the presence of tumor cells and their metabolic activity. When the positron is emitted by 18F, it annihilates a nearby electron, with the emission of two 0.511-MeV photons in opposite directions. These photons are detected by ring detectors placed in a circular gantry surrounding the patient. From the detection of these photons, a computer software (e.g., filtered back-projection algorithm) reconstructs the site of the annihilation events and the intervening anatomy. The site of increased FDG accumulation, with the surrounding anatomy, is thereby imaged with a resolution of approximately 4 mm.
Combining PET with CT scanning has several advantages:
Superior quality CT images with their geometric accuracy in defining anatomy and tissue density differences are combined with PET images to provide physiologic imaging, thereby differentiating malignant tumors from the normal tissue on the basis of their metabolic differences.
PET images may allow differentiation between benign and malignant lesions well enough in some cases to permit tumor staging.
PET scanning may be used to follow changes in tumors that occur over time and with therapy.
By using the same treatment table for a PET/CT scan, the patient is scanned by both modalities without moving (only the table is moved between scanners). This minimizes positioning errors in the scanned data sets from both units.
By fusing PET and CT scan images, the two modalities become complementary. Although PET provides physiologic information about the tumor, it lacks correlative anatomy and is inherently limited in resolution. CT, on the other hand, lacks physiologic information but provides superior images of anatomy and localization. Therefore, PET/CT provides combined images that are superior to either PET or CT images alone.
12.3. Treatment Verification
A. Port Films
The primary purpose of port filming is to verify the treatment volume under actual conditions of treatment. Although the image quality with the megavoltage x-ray beam is poorer than with the diagnostic or the simulator film, a port film is considered mandatory not only as a good clinical practice, but also as a legal record.
As a treatment record, a port film must be of sufficiently good quality so that the field boundaries can be described anatomically. However, this may not always be possible due to either very high beam energy (10 MV or higher), large source size (cobalt-60), large patient thickness (>20 cm), or poor radiographic technique. In such a case, the availability of a simulator film and/or a treatment diagram with adequate anatomic description of the field is helpful. Anatomic interpretation of a port film is helped by obtaining a full-field exposure on top of the treatment port exposure.
Radiographic technique significantly influences image quality of a port film. The choice of film and screen as well as the exposure technique is important in this regard. Droege and Bjärngard (32) have analyzed the film screen combinations commonly used for port filming at megavoltage x-ray energies. Their investigation shows that the use of a single emulsion film with the emulsion adjacent to a single lead screen3 between the film and the patient is preferable to a double emulsion film or a film with more than one screen. Thus, for optimum resolution, one needs a single emulsion film with a front lead screen and no rear screen. Conventional nonmetallic screens are not recommended at megavoltage energies. Although thicker metallic screens produce a better response, an increase in thickness beyond the maximum electron range produces no further changes in resolution (32).
Certain slow-speed films, ready packed but without a screen, can be exposed during the entire treatment duration. A therapy verification film such as Kodak XV-2 is sufficiently slow to allow an exposure of up to 200 cGy without reaching saturation. In addition, such films can be used to construct compensators for both the contours and tissue heterogeneity (33).
B. Electronic Portal Imaging
Major limitations of port films are (a) viewing is delayed because of the time required for processing, (b) it is impractical to do port films before each treatment, and (c) film image is of poor quality especially for photon energies greater than 6 MV. Electronic portal imaging overcomes the first two problems by making it possible to view the portal images instantaneously (i.e., images can be displayed on computer screen before initiating a treatment or in real time during the treatment). Portal images can also be stored on computer discs for later viewing or archiving.
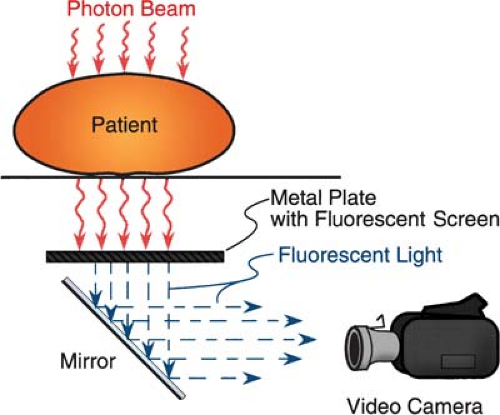
On-line electronic portal imaging devices (EPIDs) are currently being clinically used in several institutions, and some of them are commercially available. Many of the systems are video based; the beam transmitted through the patient excites a metal fluorescent screen, which is viewed by a video camera using a 45-degree mirror (34,35,36,37) (Fig. 12.13). The camera is interfaced to a microcomputer through a frame-grabber board for digitizing the video image. The images are acquired and digitized at the video rate of 30 frames per second. An appropriate number of frames is averaged to
produce a final image. Depending on the computer software, the image data can be further manipulated to improve the image quality or perform a special study.
produce a final image. Depending on the computer software, the image data can be further manipulated to improve the image quality or perform a special study.
One problem with mirror-based EPIDs is the large size of the mirror, which can pose practical problems. Wong et al. (38) have developed a system that replaces the mirror with a fiberoptics system to direct the fluorescent light to the video camera. The fiberoptics channels consist of thin clear polystyrene columns encased by a thin acrylic cladding. Because of the difference in the refractive indices of the two plastics, it is possible to conduct light without significant loss of intensity. This “light piping” is accomplished by the process of total internal reflection at the cladding interface.
Another class of EPIDs consists of a matrix of liquid ion chambers used as detectors (39,40). These devices are much more compact than the video-based systems and are comparable in size to a film cassette, albeit a little heavier. One such system developed at The Nederlands Kanker Institute consists of a matrix of 256 × 256 ion chambers containing an organic fluid and a microcomputer for image processing. Figure 12.14 shows an image obtained with such a device. Besides imaging, another potential use of this device is on-line patient dose monitoring. Further work is needed to develop this application.
Yet another type of EPID uses solid state detectors. One approach employs a scanning linear array of silicon diodes. Another uses a linear array of zinc tungstate (Zn WO4) scintillating crystals attached to photodiodes. A review of these developments is provided by Boyer et al. (41).
A variety of technologies are being explored to develop new EPIDs or refine the existing ones. For example, Varian Medical Systems has an EPID called PortalVision aS500, featuring an array of image detectors based on amorphous silicon (a-Si) technology (Fig. 12.15). Within this unit a scintillator converts the radiation beam into visible photons. The light is detected by an array of photodiodes implanted on an amorphous silicon panel. The photodiodes integrate the light into charge captures. The sensitive area of the EPID is 40 × 30 cm2 with 512 × 384 pixels, spatial resolution is
0.78 mm, and the readout image has about 200,000 pixels. This system offers better image quality than the previous system using liquid ion chambers.
0.78 mm, and the readout image has about 200,000 pixels. This system offers better image quality than the previous system using liquid ion chambers.
C. Cone-beam CT
A conventional CT scanner has a circular ring of detectors, rotating opposite an x-ray tube. However, it is possible to perform CT scans with detectors imbedded in a flat panel instead of a circular ring. CT scanning that uses this type of geometry is known as cone-beam computed tomography (CBCT).
In cone-beam CT, planar projection images are obtained from multiple directions as the source with the opposing detector panel rotates around the patient through 180 degrees or more. These multidirectional images provide sufficient information to reconstruct patient anatomy in three dimensions, including cross-sectional, sagittal, and coronal planes. A filtered back-projection algorithm is used to reconstruct the volumetric images (42).
CBCT systems are commercially available as accessories to linear accelerators. They are mounted on the accelerator gantry and can be used to acquire volumetric image data under actual treatment conditions, thus enabling localization of planned target volume and critical structures before each treatment. The system can be implemented either by using a kilovoltage x-ray source or the megavoltage therapeutic source.
C.1. Kilovoltage CBCT
Kilovoltage x-rays for a kilovoltage CBCT (kVCBCT) system are generated by a conventional x-ray tube that is mounted on a retractable arm at 90 degrees to the therapy beam direction. A flat panel of x-ray detectors is mounted opposite the x-ray tube. The imaging system thus provided is quite versatile and is capable of cone-beam CT as well 2-D radiography and fluoroscopy. All three major manufacturers of linear accelerators (Varian, Elekta, and Siemens) are offering this technology. The commercial names for these systems are Trilogy (www.varian.com), Synergy (www.elekta.com), and ONCOR (www.siemens.com). Figure 12.16 shows a picture of Elekta’s Synergy. Figure 12.17 is
an example of kVCBCT of a lung cancer patient. It should be mentioned that the accelerator-mounted imaging systems are under constant development and some advertised features may be works in progress or currently not approved by the Food and Drug Administration. The reader can get the updated information by contacting the manufacturers or visiting their web sites.
an example of kVCBCT of a lung cancer patient. It should be mentioned that the accelerator-mounted imaging systems are under constant development and some advertised features may be works in progress or currently not approved by the Food and Drug Administration. The reader can get the updated information by contacting the manufacturers or visiting their web sites.
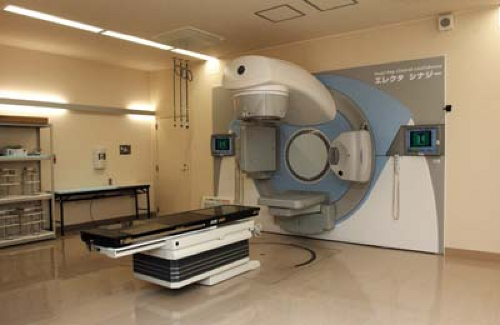 Figure 12.16. Elekta Synergy linear accelerator with on-board imaging equipment. (Courtesy of Dr. Kiyoshi Yoda, Elekta K.K., Kobe, Japan). |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree