No. of patients
Median follow-up (months)
BNI I–III relief (%)
Time to pain relief
Median dose (Gy)
Recurrence rate (%)
Complication rate (%)
Chen et al. [11] (Kaiser Los Angeles)
32
8
78
1.5 months
NR
NR
6
Richards et al. [12] (Wisconsin)
28
12
75
1 month
80
46
14
Lim et al. [13] CyberKnife (Stanford)
41
11
93
7 days
78
16
51
Smith et al. [14] (UCLA)
60
23
72
2.7 months
90
26
25
Ma from the University of Maryland performed a comparative analysis of linac and GK-SRS for TN therapy. Specifically, the dose fall-off characteristics and set-up error tolerance of the two modalities were examined. This study found equivalent dose fall-off properties between Gamma Knife and linac-based SRS provided only if a sufficient number of arcs (>7) and small intra-arc error (<0.5 mm) were satisfied for linac-based deliveries. Furthermore, because of the large number of arcs that are required, the treatment time for linac-based SRS compared with Gamma Knife is significantly increased [17]. At this time, robust safety and long-term efficacy data exist only for Gamma Knife treatment of TN; however, the clinical experience in this disease with other forms of radiosurgery is growing and will lead to greater patient options.
Mechanism of GK-SRS Effect
The relationship between post-procedure numbness and efficacy suggests that GK-SRS works by blocking axonal transmission. As predicted by models of radiation injury, both the time to effective pain relief and numbness are delayed, although pain relief frequently occurs many months before any side effects are experienced. Kondziolka et al. used a primate model to explore the effects of 80 or 100 Gy to trigeminal nerves and observed a combination of axonal degeneration and edema [18]. Necrosis was seen in nerves that received the higher dose, and both myelinated and unmyelinated fibers were equally affected. Why the functional improvement is seen in patients before these histologic changes are seen is unknown, but an effect of GK-SRS on ephaptic transmission provides a possible mechanism.
Targeting, Dose, and Dose Rate
Early SRS treatments for TN targeted the trigeminal ganglion because the trigeminal cistern in the Meckel’s cave could be inferred from landmarks on skull films, a practice later refined by stereotactic cisternography. In fact, glycerol rhizotomy was discovered incidentally during the use of glycerol as a carrier for tantalum dust in outlining the trigeminal cistern [19]. In 1991, Lindquist et al. reported 46 cases of GK-SRS directed at the trigeminal ganglion with 22 patients localized using cisternography [20]. They did not report prescription doses. The results were disappointing as only 4 of 22 patients had prolonged pain relief. Rand et al. reported a series of 12 patients treated by GK-SRS doses ranging from 57 to 74 Gy with only mild success and concluded that the ganglion was not the appropriate target [21]. Based on these findings and with the development of high-resolution MRI, which made it possible to reliably target the trigeminal nerve anywhere along its course from the pons to the Meckel’s cave, the optimal target moved toward the trigeminal nerve root entry zone.
In 1996, Kondziolka et al. published the results of a multicenter trial of GK-SRS that standardized the target [22]. A single 4-mm isocenter was applied to the trigeminal nerve 2–4 mm anterior to the junction of the nerve at the pons as seen on axial and coronal MRI scans. This location has since been confirmed effective by several large series [23]. The 20 % isocenter line typically contacts the brain stem using this protocol, which is well tolerated at the current maximum doses of 70–80 Gy to the isocenter. Evaluation of brain-stem dose as well as evidence of compression was evaluated at the University of Maryland. Doses to the brain stem ranged from 10 to 20 %, and evidence of compression was seen in 12 % of patients. Both brain-stem dose and evidence of compression did not have any effect on treatment response, medication use, or development of facial numbness [24]. Beam shaping is accomplished by plugging some of the 201 sources during a treatment and can be used to accomplish a flattening of the lower isodose curves such that the 10 % line is parallel to the brain stem (Fig. 52.1). Theoretically, this should decrease the risk of radiation necrosis to the brain stem. Some centers have also tried using two isocenters to irradiate a greater length of nerve. A randomized, prospective study reported by Flickinger et al. found no increase in efficacy but did show a statistically significant increase in trigeminal dysfunction [25]. Efficacy was reported to be 67.7 ± 5.1 % in both arms, and facial numbness and mild and severe paresthesias developed in 19 %, 12 %, and 2 % in two isocenter patients versus 7 %, 9 %, and 0% in one isocenter patient, respectively. In the multivariate analysis, the length of nerve treated was the only significant factor predicting for facial numbness.
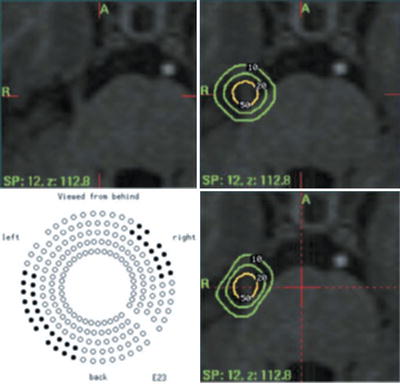

Fig. 52.1
Gamma Knife treatment plan for a patient with right trigeminal neuralgia. Isodose coverage of the nerve is seen in the right upper panel. The 20 % line touches the brain stem, and the 10 % line cuts into it. Beam shaping is accomplished by plugging 32 beams as shown in the left lower panel. The result, seen in the right lower panel, is a flattened 10 and 20 % isodose line such that the brain stem receives 10 % only along the edge
In Leksell’s original 1971 paper, he described successful treatment at maximum doses of 16.5 and 22 Gy [11]. Patients studied in the 1996 multicenter trial received doses ranging from 60 to 90 Gy [22]. Patients who received at least 70 Gy fared significantly better, but no difference was seen by increasing the dose to 90 Gy. At these doses, the edge of the brain stem receives no more than 16 Gy, which is well tolerated.
At the University of Maryland, MRI imaging is performed with gadolinium-enhanced short-TR sequences (T1-weighted) with axial volume acquisitions using 512 × 216 matrices at 1.5-mm slice intervals. Occasionally, it is difficult to visualize the affected prepontine trigeminal nerve on the axial MRI view. This was evaluated and it was found that MRI quality did not affect treatment response, medication use, and development of facial numbness [24]. Based on this study, it is recommended that if the trigeminal nerve is difficult to image, additional axial long-TR sequences (T2-weighted) or use of the coronal and sagittal views is useful (Case 52.1). A single 4-mm isocenter shot is placed just anterior to the entry of the trigeminal nerve into the pons with the 20 % isocenter barely touching the pons surface. Coronal MRI scans are examined to ensure that the 50 % line surrounds the nerve, and 90 and 99 % lines are evaluated to make sure the center of the nerve receives the maximum dose. A customized plugging pattern is then applied that elongates the lower isodose curves and minimizes the volume of brain stem covered by the 10 % line. Finally, a dose of 75 Gy is delivered to the 100 % isocenter (37.5 Gy to the 50 %).
Case 52.1
This 42-year-old man with a 10-year history of well-controlled multiple sclerosis began experiencing typical right V3 trigeminal neuralgia 4 years prior to treatment. He had been placed on increasing doses of Tegretol and Neurontin with decreasing effectiveness. The increased doses of medication also worsened his MS symptoms. He had typical lancinating pain with no numbness that was triggered by touching the face and talking. At the time of GK-SRS, he had T1- and T2-weighted MRI scans done. Because the T1 scans did not show the nerve, we used the T2 scan to target the nerve (Fig. 52.2a, b). A 4-mm shot was used with a plugging pattern to shape the 10 and 20 % isodose lines. A dose of 75 Gy was delivered to the 100 % line. One month after GK-SRS, he noticed the sudden improvement in his pain. At 2 months after treatment, he was weaned off both medications. He remains pain-free for 2 years.
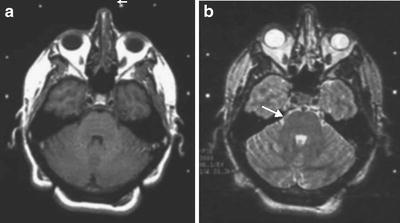

Fig. 52.2
MRI images from the Gamma Knife treatment of a patient with right trigeminal neuralgia. (a) T1-weighted MRI scan that shows the left nerve (arrow) but not the right. (b) T2-weighted MRI scan at the same level illustrates the right nerve well (arrow)
Patients with a pacemaker or other MRI contraindications present a dilemma. Provided the patient is not on anticoagulants, computed tomography (CT) contrast cisternography is used for localizing the trigeminal nerve (Case 52.2). In rare circumstances, an MRI scan in patients with a pacemaker is required. A cardiologist is present in these cases in the MRI suite with a defibrillator, and the pacemaker is programmed before the scan to avoid extraventricular pacing and is then reset immediately after the MRI.
Case 52.2
This 60-year-old man had a 5-year history of typical trigeminal neuralgia that initially responded to glycerol rhizotomy. His pain recurred and he required treatment with Tegretol, which was becoming ineffective. One year prior to GK-SRS, he had a cardiac defibrillator placed for arrhythmias. A cisternogram was performed through a C1–2 puncture. Axial CT scans were obtained that showed the right trigeminal nerve (Fig. 52.3). The nerve was targeted according to protocol with a 4-mm shot of radiation with a total dose of 75 Gy delivered to the isocenter. Three weeks after treatment, his pain disappeared. He remains pain-free 1 year after treatment.
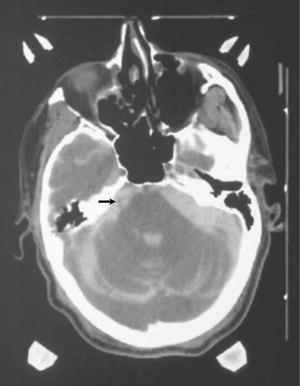

Fig. 52.3
CT cisternogram of a patient with right trigeminal neuralgia and a cardiac defibrillator. CT contrast in the CSF cisterns around the trigeminal nerve highlights it well (arrow)
For gamma rays, dose rate is one of the principal factors that determine the biologic consequences of a given absorbed dose. As the dose rate is lowered and the exposure time extended, the biologic effect of a given dose is generally reduced. The University of Maryland examined this effect in a series of patients treated the year prior to a source change (median dose rate, 162 cGy/min: range, 151 to 179 cGy/min) compared with patients treated in the year immediately after a source change (median dose rate, 343 cGy/min: range, 321 to 366 cGy/min) and found the difference in pain relief was 61 % compared with 83 %, respectively (p = 0.05). These data suggest that if dose rate falls below 179 cGy/min, consideration should be made for dose escalation in the treatment of TN [26].
Treatment Response
Assessing positive treatment responses in TN is challenging because the symptoms are subjective. The BNI pain intensity score combines severity of pain with medication use (Table 52.2) [27]. Patients able to stop all medications are classified as BNI I if they have complete pain relief or BNI II if they have occasional tolerable pain. Patients remaining on medication are BNI III if they have good pain relief, but are BNI IV if they are not adequately controlled. Patients receiving no relief are classified BNI V. Most patients are BNI V before their treatment. For comparison with other series, we consider BNI I to III to be a good outcome. The McGill pain score is useful in the preoperative patient assessment (Table 52.3). Patients are asked to rate their TN pain and their worst non-TN headache using a range from mild (I) to excruciating (V).
Table 52.2
Barrow Neurological Institute pain intensity score
I | No trigeminal pain, no medications |
II | Occasional pain that is well tolerated, no medications |
III | Occasional pain that requires medication to be tolerated |
IV | Pain that is not adequately controlled with medication |
V | Severe pain without relief |
Table 52.3
McGill pain assessment score
I | Mild |
II | Discomforting |
III | Distressing |
IV | Horrible |
V | Excruciating |
A comparison of selected major series reported in the literature is shown in Table 52.4 [22, 23, 27–32]. In general, there is good correlation between the different series. The range of patients achieving good relief (BNI I to III) is 69–94 % with 22–76 % achieving complete pain relief on no medications (BNI I). The median time to pain relief varies from 2 to 4 weeks. An actuarial analysis of recurrence has shown rates of 23 %, 33 %, and 39 % at 1, 2, and 3 years, respectively [28]. A follow-up of the original Maryland series (median follow-up 5.6 years) shows that recurrent pain become more common over time. At 5 and 7 years after treatment, only 34 and 22 % of patients were free of recurrence [33]. Quality of life assessments have documented that most patients believe their GK-SRS treatments were successful even when the relief was temporary. The lack of side effects appears significant in this regard, and a high proportion of patients who fail initial GK-SRS elect for an additional treatment.
Table 52.4
Results of initial GK-SRS in idiopathic TN
No. of patients | Median follow-up | BNI I relief (%) | BNI I–III relief (%) | Time to pain relief (weeks) | Median dose | Recurrence rate (%) | Complication rate (%) | |
|---|---|---|---|---|---|---|---|---|
Kondziolka et al. [22] (multicenter) | 50 | 18 months | 58 | 94 | 4 | 70 Gy to the 100 % | 6 | 10 |
Maesawa et al. [23] (University of Pittsburgh) | 220 | 22 months | 40 | 69 | 8 | 80 Gy to the 100 % | 13.6 | 7.7 |
Rogers et al. [27] (Barrow Neurological Institute) | 54 | 12 months | 35 | 89 | 2 | 35 Gy prescribed to the 50 % | 21 | 9 |
Petit et al. [28] (University of Maryland)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





