CHAPTER 45 Truncus Arteriosus
Truncus arteriosus is an uncommon but potentially lethal congenital heart disease that manifests during the neonatal period or early infancy. It is defined by a common origin of the aorta and the pulmonary arteries, resulting from an incomplete embryologic septation and separation of the aorta and the pulmonary trunk. This congenital cardiac anomaly was first described in 1798 by Wilson.1 In 1942, Lev and Saphir2 proposed the anatomic criteria that defined truncus arteriosus. Since then, different classifications had been proposed by Collett and Edwards3 in 1949, by Van Praagh and Van Praagh4 in 1965, and by the Society of Thoracic Surgeons in 2000.5
CLASSIFICATIONS
Historically, two major classification schemes for anatomic variations of the truncus arteriosus were proposed by Collett and Edwards3 and by Van Praagh and Van Praagh.4 The Collett and Edwards classification is strictly defined by the anatomic origins of the pulmonary arteries. It divides truncus arteriosus into four types, I to IV (Fig. 45-1). In Collett and Edwards type I, a common arterial trunk divides into an aorta and a short pulmonary trunk, which divides into the left and right branch pulmonary arteries. This is the most common configuration (48% to 68% of cases). In Collett and Edwards type II (29% to 48% of cases), the left and right branch pulmonary arteries arise close together from the common arterial trunk without a distinct pulmonary trunk. Collett and Edwards type III (6% to 10% of cases) is similar to type II except that the origins of the pulmonary arteries are far apart. Collett and Edwards described a type IV configuration in which the pulmonary arteries arise from the descending aorta. These arteries are now known to be major aorticopulmonary collateral arteries associated with pulmonary atresia with ventricular septal defect (VSD) and not truncus arteriosus.
The Collett and Edwards classification developed in 1949 did not consider the roles of patent ductus arteriosus (PDA) and interrupted aortic arch, which are associated with truncus arteriosus and have prognostic and surgical implications. Subsequently, Van Praagh and Van Praagh4 proposed a different classification with four types, 1 to 4 (Fig. 45-2). Each number may have a prefix letter A if a VSD is present (common) or B if a VSD is absent (rare). Van Praagh and Van Praagh type 1 is identical to Collett and Edwards type I, describing a short pulmonary trunk arising from the common arterial trunk. Van Praagh and Van Praagh type 2 describes separate origins of the left and right branch pulmonary arteries arising from the common arterial trunk, regardless of the distance separating their origins (Fig. 45-3). It corresponds to Collett and Edwards type II and type III. Van Praagh and Van Praagh type 3 describes one branch pulmonary artery arising from the common arterial trunk and the other connected to a ductus arteriosus or an aorticopulmonary collateral artery (Fig. 45-4). Van Praagh and Van Praagh type 4 describes the coexistence of a common arterial trunk and an interrupted or a severely hypoplastic aortic arch. The descending aorta is supplied by a PDA. Van Praagh and Van Praagh type 3 and type 4 have no correspondence in the Collett and Edwards classification.
Although the Van Praagh and Van Praagh classification provides a more refined description of truncus arteriosus, it does not completely serve the needs of surgeons. In 2000, the Society of Thoracic Surgeons proposed a uniform reporting system with modifiers that better describe anatomic features useful for surgical outcome studies.5 In this system, cryptic numerical labels are replaced by descriptive phrases. Three major types are described: truncus arteriosus with confluent or near-confluent pulmonary arteries (corresponding to Van Praagh and Van Praagh type 1 and type 2), truncus arteriosus with absence of one pulmonary artery (corresponding to Van Praagh and Van Praagh type 3), and truncus arteriosus with interrupted aortic arch or coarctation (corresponding to Van Praagh and Van Praagh type 4). Additional modifiers describe the presence or absence of VSD, the number of truncal leaflets, the presence of truncal insufficiency or stenosis or both, coexisting coronary anomalies, any ventricular hypoplasia, the truncal valve position relative to the ventricles, and the presence or absence of thymus.
EPIDEMIOLOGY AND GENETICS
Truncus arteriosus occurs in 1% to 2% of infants born with congenital heart defects, or about 10 cases per 100,000 live births.6 Among conotruncal anomalies, truncus arteriosus has a similar rate of occurrence as congenitally corrected transposition of the great arteries and double outlet right ventricle, but it is three times less common than complete transposition of the great arteries and four times less common than tetralogy of Fallot. There is no predilection by sex. Of patients with truncus arteriosus, 34% to 41% harbor a chromosome 22q11 deletion.7 This deletion is also seen in a large proportion of patients with conotruncal developmental anomalies as found in DiGeorge syndrome, velocardiofacial syndrome, and interrupted aortic arch, suggesting the importance of this gene in the regulation of the embryologic development of the conotruncus. Today, this chromosomal deletion is readily detected with the fluorescence in situ hybridization technique.
Genetic screening is important because 22q11 deletion is inherited in an autosomal dominant fashion from one parent in 6% to 28% of cases.8 This is important information for genetic family counseling. Knowledge of this deletion also heightens clinical suspicions for associated anomalies, including athymia, hypocalcemia, and nasopalatal malformation. Truncus arteriosus is also associated with trisomy 8 and chromosomal 10p deletion.9
ETIOLOGY AND PATHOPHYSIOLOGY
Embryology
In normal embryologic development, beginning in the fifth week of gestation, fusion of the endocardial cushions separates the atrioventricular canal into right and left openings, which are destined to become the tricuspid annulus and the mitral annulus. Through these two openings, blood flow develops into two intertwined, spiraling streams that flow out the conus cordis and the truncus arteriosus, or conotruncus for short. Between the two spiraling streams is a layer of stagnant blood. There is evidence that this layer of stagnant blood promotes the development of two ridges of tissue at opposing margins inside the conotruncus.10 These two ridges grow toward each other until they meet and fuse. The fusion begins at the conotruncal junction. Similar to the closing of a zipper, the fusion proceeds upward through the truncus arteriosus, creating two spiraling lumens that become the ascending aorta and the pulmonary trunk. From this junction, the fusion also travels downward through the conus cordis until it meets the ventricular septum, separating the right and left ventricles. Separation of the systemic and the pulmonary circulations is completed by 9 weeks of gestation. Beginning in the seventh week, specialized tissue swellings from the conotruncal junction evolve into the aortic and the pulmonary semilunar valves; these are also completed by 9 weeks.
Associated Cardiac Anomalies
Because the conus cordis is not partitioned properly below the truncal valve, a VSD usually exists at the infundibular septum beneath the truncal valve (Fig. 45-5). This VSD is often large and nonrestrictive. The right ventricle, subjected to the systemic pressure generated by the left ventricle, becomes hypertrophic. In most instances (68% to 83%), the common arterial trunk and the truncal valve straddle the ventricular septum in a manner resembling the overriding aorta in tetralogy of Fallot or pulmonary atresia with VSD. Uncommonly (11% to 29%), the truncal valve aligns exclusively with the right ventricle. Rarely (4% to 6%), it aligns with the left ventricle. In the two latter situations, the VSD may be small or absent.
The truncal valve may have one to five leaflets. Tricuspid truncal valve (Fig. 45-6A) is the most common (69%), followed by quadricuspid valve (22%) (see Fig. 45-6B
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


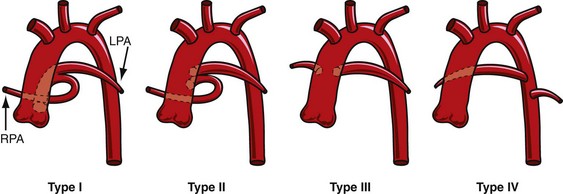
 FIGURE 45-1
FIGURE 45-1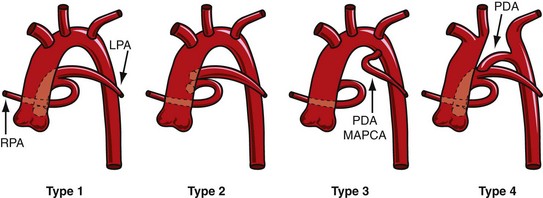
 FIGURE 45-2
FIGURE 45-2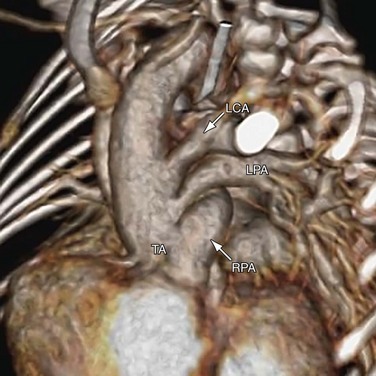
 FIGURE 45-3
FIGURE 45-3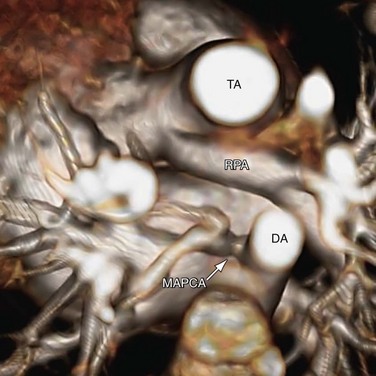
 FIGURE 45-4
FIGURE 45-4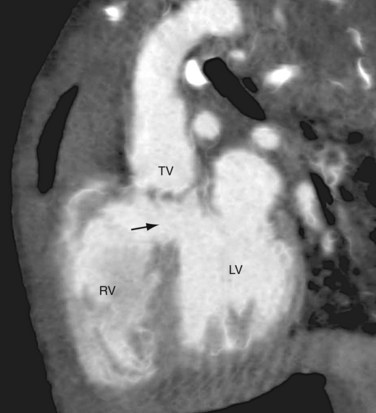
 FIGURE 45-5
FIGURE 45-5