There are several tumors and tumorlike masses involving multiple spaces in the pediatric brain. Accurate diagnosis of tumors and distinguishing them from tumorlike masses is an important aspect in the diagnostic workup and plays a key role for management and prognosis. Neuroimaging plays an important role in (1) identification of a brain mass, (2) determining its location, (3) demonstrating involvement of a single space versus multiple spaces, and (4) distinguishing a tumor from tumorlike masses.
Key points
- •
The pediatric central nervous system is affected by tumors and tumorlike masses that involve multiple spaces.
- •
Neuroimaging plays an important role in distinguishing tumors from tumorlike masses.
- •
MR imaging may represent the best predictor of neurodevelopmental abnormalities, seizures, and requirement of neurosurgery in neurocutaneous melanosis.
- •
Histiocytic disorders, infectious or inflammatory processes, and neurocutaneous syndromes should be considered potential tumor mimics.
Introduction
Brain tumors are the most common solid tumor of childhood, the second most common malignancy overall after leukemia and the leading cause of mortality among all childhood cancers. Primary brain tumors have been categorized by the World Health Organization classification to include tumors of the neuroepithelium, cranial nerves, meninges, and sella and those of hematopoietic and germ cell origin. Brain tumors typically form masses, which infiltrate or compress brain parenchyma. The tentorium cerebelli divides the brain parenchyma into supratentorial and infratentorial compartments. The meningeal layers covering the brain create potential anatomic spaces such as epidural, subdural, and subarachnoid. Masses may be confined to a single compartment/space or may extend across multiple compartments/spaces. Not every brain mass, however, represents a tumor or neoplasm. Tumorlike masses of the pediatric brain include histiocytic disorders, inflammatory lesions, vasculitis, ischemia, hemorrhage, vascular malformations, and malformations of brain development.
The recognition of a brain mass on imaging is the first step in the workup. This recognition is followed by verifying its location, identifying it as a tumor and distinguishing it from tumorlike mimics, characterizing the tumor grade and type, and describing its characteristics (including vascularity, vascular permeability, and metabolic spectrum). Hence, imaging plays a vital role in establishing the correct diagnosis, establishing a therapeutic strategy, and influencing prognosis. MR imaging, including conventional (anatomic) and advanced (functional) sequences, is the study of choice.
This article reviews pediatric brain tumors that involve multiple spaces, including lymphoproliferative disorders, melanocytic lesions, metastasis, tumors of meningothelial cells, and tumors in children with neurofibromatosis type 1 (NF-1), neurofibromatosis type 2 (NF-2), and von Hippel-Lindau (VHL) disease. In addition, tumorlike masses, which include histiocytic disorders, inflammatory processes, infectious etiologies, and neurocutaneous syndromes, are reviewed. For each entity, epidemiology, pathophysiology, and neuroimaging findings are discussed. For tumorlike masses, features and findings that allow the correct differentiation from neoplastic masses are discussed.
Introduction
Brain tumors are the most common solid tumor of childhood, the second most common malignancy overall after leukemia and the leading cause of mortality among all childhood cancers. Primary brain tumors have been categorized by the World Health Organization classification to include tumors of the neuroepithelium, cranial nerves, meninges, and sella and those of hematopoietic and germ cell origin. Brain tumors typically form masses, which infiltrate or compress brain parenchyma. The tentorium cerebelli divides the brain parenchyma into supratentorial and infratentorial compartments. The meningeal layers covering the brain create potential anatomic spaces such as epidural, subdural, and subarachnoid. Masses may be confined to a single compartment/space or may extend across multiple compartments/spaces. Not every brain mass, however, represents a tumor or neoplasm. Tumorlike masses of the pediatric brain include histiocytic disorders, inflammatory lesions, vasculitis, ischemia, hemorrhage, vascular malformations, and malformations of brain development.
The recognition of a brain mass on imaging is the first step in the workup. This recognition is followed by verifying its location, identifying it as a tumor and distinguishing it from tumorlike mimics, characterizing the tumor grade and type, and describing its characteristics (including vascularity, vascular permeability, and metabolic spectrum). Hence, imaging plays a vital role in establishing the correct diagnosis, establishing a therapeutic strategy, and influencing prognosis. MR imaging, including conventional (anatomic) and advanced (functional) sequences, is the study of choice.
This article reviews pediatric brain tumors that involve multiple spaces, including lymphoproliferative disorders, melanocytic lesions, metastasis, tumors of meningothelial cells, and tumors in children with neurofibromatosis type 1 (NF-1), neurofibromatosis type 2 (NF-2), and von Hippel-Lindau (VHL) disease. In addition, tumorlike masses, which include histiocytic disorders, inflammatory processes, infectious etiologies, and neurocutaneous syndromes, are reviewed. For each entity, epidemiology, pathophysiology, and neuroimaging findings are discussed. For tumorlike masses, features and findings that allow the correct differentiation from neoplastic masses are discussed.
Tumors across multiple spaces
Lymphoproliferative Disorders
Lymphoproliferative disorders in childhood include central nervous system (CNS) involvement in childhood leukemia and non-Hodgkin lymphoma (NHL), primary central nervous system lymphoma (PCNSL), and posttransplant lymphoproliferative disorder with CNS involvement (CNS PTLD). Lymphoproliferative disorders may extend across multiple spaces, such as the brain parenchyma, meninges, bone marrow, or calvarium.
Central nervous system involvement in childhood leukemia
Acute lymphoblastic leukemia (ALL) is a common hematologic malignancy of the bone marrow in which precursor lymphoblasts, blocked at an early stage of differentiation, proliferate rapidly and replace normal hematopoietic cells of the bone marrow. ALL is the most common childhood malignancy, accounting for approximately 25% of cancers and 80% of all leukemias in children.
CNS involvement is typically clinically occult and discovered at the time of lumbar puncture. Children may present with cranial nerve deficits, seizure, altered mental status, headache, or other neurologic deficits.
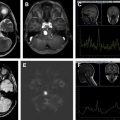
Primary neuroimaging manifestations of ALL include enlargement of the lateral ventricles (present before therapy, hence, representing hydrocephalus rather than atrophy), leukemic infiltration of CNS structures (meninges, parenchyma, bone marrow, orbit, and spine; Fig. 1 A–D ), and cerebrovascular complications (hemorrhage, cerebral infarction).
Leukemic infiltration may involve the leptomeninges, subarachnoid space, or epidural space. Meningeal thickening and enhancement can be either focal or diffuse on postcontrast T1-weighted images. Leptomeningeal enhancement of the brain may result from CNS leukemia/relapse, infection, or, rarely, both. Parenchymal leukemic infiltration is very uncommon, it is often hyperdense on CT, it is contiguous with a meningeal surface, and it enhances after contrast material administration. On MR imaging, parenchymal leukemic CNS infiltration tends to be slightly hypointense on T1-weighted images and isointense to mildly hyperintense on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images compared with gray matter.
Chloroma, also known as granulocytic sarcoma , is a focal extramedullary mass of immature myeloid cells of granulocytic lineage that infiltrate bone and soft tissue, most frequently occurring in myelogenous leukemia. Chloromas most commonly arise in the skull, orbits, and sinuses ( Fig. 2 ).
Spinal involvement is frequent in leukemia and lymphoma, either as initial manifestation or in relapse, with bone marrow or meningeal infiltration ( Fig. 1 E, F). MR imaging of the bone marrow shows low-signal-intensity leukemic infiltrates on T1-weighted images. Subarachnoid nodules, enhancement, and sugar coating of the nerve roots and cauda equina consistent with leptomeningeal seeding are seen on contrast-enhanced T1-weighted images.
Primary cerebrovascular complications are related to leukocytosis, thrombocytopenia, sepsis, or coagulopathy. Hemorrhage is the most common cerebrovascular complication. Hemorrhage can be identified on conventional MR imaging sequences such as T1 and T2 and on advanced sequences such as susceptibility-weighted imaging. Hemorrhage may show mass effect, midline shift, and perifocal edema.
More common than primary leukemic infiltration and primary cerebrovascular complications are treatment-related changes of the CNS including infection, acute neurotoxicity, demyelination, and hemorrhage.
Central nervous system involvement in childhood non-Hodgkin lymphoma
CNS involvement in NHL is identified by either malignant cells in the cerebrospinal fluid or by cranial nerve palsy on physical examination, which guides further management. About 6% of childhood/adolescent NHL patients are CNS positive during the course of the disease. On MR imaging, leptomeningeal disease with meningeal thickening and enhancement may be seen. MR imaging is highly sensitive for the presence of meningeal pathologic conditions, but nonspecific for the disease entity. For example, benign meningeal enhancement may be observed after diagnostic or therapeutic lumbar puncture. Soft tissue masses arising from the maxillofacial or pharynx may have trans-spatial extension to the skull base or intracranial compartment ( Fig. 3 ). In addition, parenchymal mass lesions similar to PCNSL may be seen.
Primary central nervous system lymphoma
PCNSL represents a rare subtype of NHL restricted to the CNS. Children with immune deficiency syndromes are at increased risk for PCNSL development; however, most reported cases occur in immunocompetent patients. In PCNSL, lesions are typically multiple and located within the brain parenchyma. Deep brain structures such as basal ganglia, thalami, cerebellum, and brainstem are most commonly involved, whereas infiltration of the cerebral hemispheres and isolated meningeal involvement are less frequent. Lesions are typically isointense to hyperintense on T2-weighted images and hypointense or isointense on unenhanced T1-weighted MR images. Enhancement is shown after administration of contrast. Because of tumor hypercellularity, water diffusion is often restricted, making them appear hyperintense on trace of diffusion maps (diffusion-weighted imaging [DWI]) with matching low apparent diffusion coefficient values. Microhemorrhages and calcifications are rare in PCNSL, thus, differentiating them from high-grade gliomas.
Posttransplant lymphoproliferative disorder with central nervous system involvement
CNS PTLD in children is uncommon and can prove diagnostically challenging. Lung, liver, heart, and intestinal transplant patients have a higher risk of CNS PTLD development compared with kidney and bone marrow allograft recipients. Multifocal parenchymal lesions are typically seen in CNS PTLD with MR imaging characteristics similar to those of PCNSL. However, intraparenchymal hemorrhage is an uncommon feature of PCNSL and hence a potential distinguishing feature.
Primary Melanocytic Lesions
Congenital melanocytic nevi (CMN) can be single or multiple and are present at birth or arise within the first few weeks of life. Multiple CMN can be associated with neurologic abnormalities of the CNS, traditionally termed neurocutaneous melanosis (NCM). NCM (OMIM 249400 ) is caused by somatic mutation in the NRAS gene on chromosome 1p13 and is defined by the presence of multiple (>3) or giant CMN and infiltration of brain or leptomeninges by abnormal melanin deposits.
In children with 2 or more CMN at birth, MR Imaging of the neural axis is the modality of choice to search for CNS involvement and should be performed in the first year of life (ideally within the first 6 months because of myelination obscuring the signal from melanin). Myelination may obscure melanin deposits beyond the age of 6 months, as both myelin and melanin appear bright on T1-weighted MR images and hypointense on T2-weighted MR images. MR imaging is the best predictor of neurodevelopmental abnormalities, seizures, and requirement for neurosurgery in childhood. MR imaging neither rules out nor predicts those who will ultimately have symptoms. A total of 60% to 70% of patients with NCM are symptomatic.
Melanin, methemoglobin, lipid, protein, calcium, iron, copper, and manganese are a few substances that appear hyperintense on T1-weighted images. In NCM, the typical location for melanin deposition is in the anterior regions of the temporal lobes (amygdala), cerebellum, pons, and leptomeninges ( Fig. 4 A ). When melanosis is present around the cerebellum and pons, hypoplasia of the cerebellum (primarily of the vermis) and pons is typically seen, and the condition may be misdiagnosed as pontocerebellar hypoplasia. The fourth ventricle may be enlarged mimicking a Dandy-Walker malformation. In addition to T1 hyperintensity on the precontrast images, diffuse nodular enhancement may be present postcontrast with progressive leptomeningeal melanocytosis ( Fig. 4 B). Progressive leptomeningeal melanocytosis or melanoma (defined by progressive growth of the lesion, presence of surrounding edema or mass effect, or presence of central necrosis) of the brain and spine is associated with a poor prognosis. Proliferating leptomeningeal deposits are associated with uncontrolled communicating hydrocephalus and or increasing intracranial pressure (see Fig. 4 B). Spinal arachnoid cysts are a common finding in children with NCM.
Metastasis
The CNS is a rare site of metastasis from a solid extracranial primary tumor in children. The most common solid primary tumors reported in children are sarcomas, neuroblastomas, nephroblastomas, and germ cell tumors. Metastasis may be in an intradural or extradural location. Intradural lesions may involve the leptomeninges, brain, or spinal cord, and are typically of hematogenous spread. Extradural lesions typically arise from contiguous structures such as bone, skin, or soft tissues. Extradural lesions may also be reached by venous plexus or lymph channels. MR imaging is the study of choice to search for metastasis. Metastasis may be multiple and extend across multiple compartments ( Fig. 5 ). Neuroimaging features of brain metastasis include perifocal edema, mass effect, and avid contrast enhancement. Metastases are subcortical- or cortical-based lesions, which are T1 isointense and T2 hyperintense.
Meningothelial Cell Lesions
Meningiomas in children account for less than 3% of all primary CNS tumors with a slight male predominance. Although meningiomas represent the most common dura-based neoplasm in the pediatric population, they have a predilection for occurring in unusual sites such as intraventricular and infratentorial regions. Meningiomas can be characterized into 3 clinical groups: (1) spontaneously arising meningiomas, (2) NF-2-associated meningiomas, and (3) radiation-induced meningiomas. Multiplicity of meningiomas is associated with NF-2 ( Fig. 6 ) and postradiation. Cystic components in meningiomas are more commonly found in children than in adults. In addition, pediatric meningiomas are often large, grow rapidly, and are more frequently malignant compared with meningiomas in adults.
Typical MR signal characteristics of meningiomas are (1) isointense to gray matter on T1-weighted images, (2) isointense to hyperintense to gray matter on T2-weighted and FLAIR images, (3) heterogeneous postcontrast enhancement, (4) calcification, (5) peritumoral edema, (5) bone erosion, and (6) hyperostosis. Dural tail is typically seen in radiation induced tumors and NF-2. Perfusion-weighted imaging may be helpful in differentiating meningeal tumors from neuroepithelial tumors because the last ones show persistence of abnormal relaxivity after the bolus of contrast because of the absence of the blood–brain barrier.
Neurofibromatosis Type 1
NF-1 (OMIM 162200 ) is the most common neurocutaneous syndrome with a prevalence of 1 in 2500 to 3000 individuals. It is an autosomal dominant disorder caused by heterozygous mutation in the neurofibromin gene on chromosome 17q11.2. Neurofibromin is widely expressed with high levels in the nervous system and acts as a tumor suppressor. Neurofibromin reduces cell growth and proliferation by negative regulation of the cellular proto-oncogene p21RAS and by control of the serine threonine kinase mTOR (mammalian target of rapamycin). Impaired neurofibromin function predisposes to benign and malignant tumor formation.
The main clinical manifestations of NF-1 involve the skin and the nervous system, but the complications are variable and may involve most of the body systems.
Neuroimaging abnormalities in NF-1 include intracranial neoplasms, parenchymal T2- hyperintense lesions, cerebral vasculopathy, and sphenoid wing dysplasia. Intracranial neoplasms include glioma and plexiform neurofibroma. Gliomas generally develop in different spaces/compartments including the optic pathways, brainstem, and, rarely, cerebellum. Optic pathway gliomas are the most frequent neoplasms seen in about 15% of children with NF-1 and are typically low-grade pilocytic astrocytomas. Optic pathway gliomas appear as concentrically enlarged irregular optic nerves or an enlarged optic chiasm, with T2-hyperintense signal ( Fig. 7 ). The tumor may extend from the intraorbital compartment to the chiasm and the optic pathways. The degree of gadolinium contrast enhancement does not correlate with tumor grade.