Tumor Response To Primary Chemotherapy
Eva C. Gombos, MD
Terminology
Abbreviations
Neoadjuvant chemotherapy (NAT), primary chemotherapy
Response evaluation criteria in solid tumors (RECIST)
Residual disease (RD)
Pathologic complete response (pCR)
Residual cancer burden score (RCB)
RCB-0 (no RD), RCB-I (little RD)
RCB-II (moderate RD), RCB-III (extensive RD)
Definitions
NAT: Systemic chemotherapy ± hormonal treatment of breast cancer prior to definitive surgery
Proposed guidelines for evaluating response
World Health Organization (WHO)
Bidimensional method: Product of longest diameter and that perpendicular to it, summed over all measured tumors
RECIST by international working group
Unidimensional measurements: Sum of longest diameters
Pathologic response: Gold standard
pCR = No residual invasive tumor; complete tumoral regression
Partial (WHO: 50% or more tumor reduction; RECIST: 30% or more reduction from baseline)
Stable disease, poor (no) response
Miller and Payne grading 1 through 5
Grade 5 = no invasive cells are identifiable (pCR)
Grade 1 = no reduction in overall number of malignant cells (nonresponder)
Adjuvant therapy: Chemotherapy ± hormonal treatment following definitive breast surgery
Anatomy-Based Imaging Issues
Overview
NAT: Common approach for treating large primary breast cancers
Increasing use with operable palpable breast cancers
Ideal opportunity to observe response of tumor in vivo, possible to distinguish responders from nonresponders
High-grade tumors, ER negative tumors, and circumscribed masses often have good response
MR during &/or at conclusion of treatment to assess response
Predicts residual tumor size
Research results of MR spectroscopy (MRS) and diffusion weighted imaging (DWI) are promising
Establish extent of disease prior to initiation of treatment
Evaluate contralateral breast for occult disease
Core biopsy with clip to establish diagnosis, receptor studies
FNA of suspicious node or sentinel node dissection
Metastases to axillary lymph nodes: Most important prognostic factor before and after NAT
Complete pathologic response predicts significantly better outcome
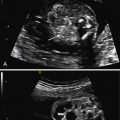
MR Features
MR Patterns of Large Breast Cancers & Histologic Correlation
Advanced MR Techniques
DWI: High cellularity may show decreased diffusion
MRS: Choline (cancer metabolite) resonance identification within tumor
Interstitial fluid pressure (IFP): Cancers found to have much higher IFP than normal tissue
Differential Diagnosis
Fibrosis (Scar, Inflammation)
May enhance: Mimics residual tumor
Residual Tumor
Decrease in enhancement on MR may be present despite viable tumor cells
Often found at pathology as scattered nests of cells
Implications and Management
Prognosis
Pathologic response is predictive of patient survival
Selected References
1. Hylton N: MR imaging for assessment of breast cancer response to neoadjuvant chemotherapy. Magn Reson Imaging Clin N Am. 14(3):383-9, vii, 2006
2. Sachelarie I et al: Primary systemic therapy of breast cancer. Oncologist. 11(6):574-89, 2006
3. Demartini WB et al: Computer-aided detection applied to breast MRI: assessment of CAD-generated enhancement and tumor sizes in breast cancers before and after neoadjuvant chemotherapy. Acad Radiol. 12(7):806-14, 2005
4. Partridge SC et al: MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol. 184(6):1774-81, 2005
5. Murata Y et al: Utility of initial MRI for predicting extent of residual disease after neoadjuvant chemotherapy: analysis of 70 breast cancer patients. Oncol Rep. 12(6):1257-62, 2004
6. Rosen EL et al: Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 181(5):1275-82, 2003
7. Esserman L et al: MRI phenotype is associated with response to doxorubicin and cyclophosphamide neoadjuvant chemotherapy in stage III breast cancer. Ann Surg Oncol. 8(6):549-59, 2001
Image Gallery
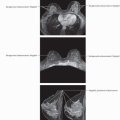
RESIDUAL NMLE: RESIDUAL DCIS ONLY: PCR
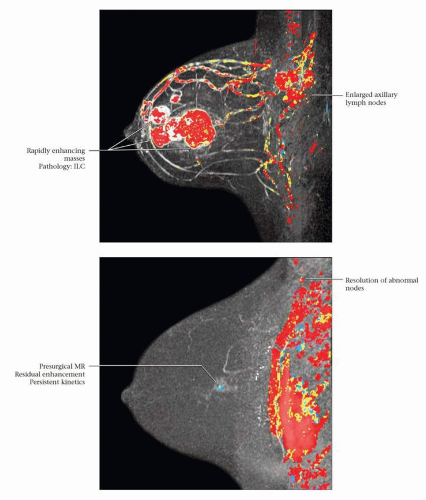 (Top) 41-year-old woman with no risk factors and a new left breast palpable mass. Mammography and US revealed multiple masses; US-guided CNB showed ER(-), PR(-), Her-2/neu(+), pleomorphic ILC. Color MIP image from MR, performed to document pretreatment extent of disease, shows multiple masses and enlarged axillary nodes; FNA of nodes was positive for malignancy. (Bottom) At the conclusion of treatment, there was minimal residual enhancement in the posterior aspect of the largest tumor site. Pathology at lumpectomy showed no invasive cancer, only residual DCIS in the tumor bed. Complete response (pCR) can be assigned at pathology if no invasive cancer is detected, regardless of the presence of DCIS (tumor bed identified). At axillary dissection 31 nodes were negative. LN positivity prior to treatment allows establishing a complete LN response post-treatment. |
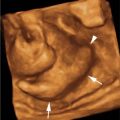
RESIDUAL NMLE: PCR
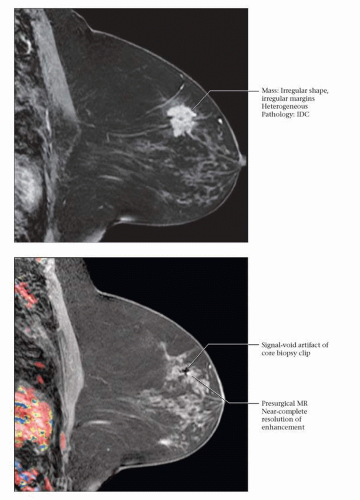 (Top) 56 year old with known BRCA2 mutation and a recent diagnosis of LVI positive, triple negative, grade II-III IDC in the left breast. MR performed prior to primary chemotherapy shows an irregular mass with spiculated margins and heterogeneous internal enhancement. (Bottom) The patient underwent cisplatin and Avastin neoadjuvant treatment. Post-treatment, preoperative MR shows no residual mass and near-complete resolution of the enhancement around the clip placed at the time of core biopsy. The patient opted for bilateral mastectomies and immediate implant reconstruction (prophylactic mastectomy on the right and left total mastectomy for treatment). Left mastectomy specimen revealed no residual invasive tumor or DCIS at the area of fibrosis, defining a region of complete tumoral regression: Miller-Payne grade 5 response. |
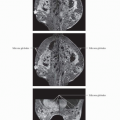
RADIOLOGIC AND PATHOLOGIC CR
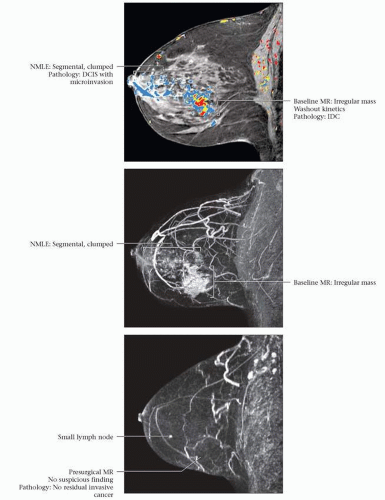 (Top) 57-year-old woman with no known risk factors had a left breast palpable mass. Mammography and ultrasound revealed a lower outer quadrant mass and US-guided CNB showed ER(-), PR(-), Her-2/neu(3+), grade III IDC and DCIS with multiple foci of microinvasion. The 1st MR was performed to document the pretreatment extent of disease. Angiomap shows the known large cancer and surrounding segmental clumped NMLE. FNA of an axillary lymph node was positive for malignancy. (Middle) MIP image shows the tumor, adjacent NMLE, and prominent tumor vascularity. (Bottom) MR at completion of NAT shows no abnormal enhancement. Pathology at mastectomy revealed no residual invasive cancer, however, DCIS was present in 6 out of 18 blocks (Miller-Payne grade 5 response). Fourteen axillary nodes were free of carcinoma. The patient is disease free at 2-year follow-up.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|