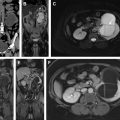
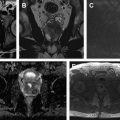
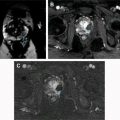
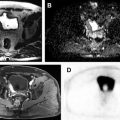
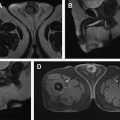
Hematuria evaluation remains a common problem, particularly in patients who smoke and are at risk for urothelial tumors. Lifetime surveillance of the urothelium is often required once urothelial cancer is diagnosed. Computed tomography urography (CTU) has exquisite sensitivity and specificity for identification of renal and urothelial lesions. The examination is well accepted by patients and physicians. Possible harms include radiation exposure and contrast-induced nephropathy. MR imaging is also an accurate test, but requires longer exam times, and may not demonstrate stones. We present the technical and interpretation skills required to use MR urography and CTU effectively.
Key points
- •
Computed tomography (CT) urography is the single best examination for comprehensive evaluation of the upper urinary tract.
- •
MR urography is an appropriate substitute for CT urography when there are contraindications to CT, including both ionizing radiation (young patients, pregnancy, serial examinations) and iodinated contrast (renal insufficiency, allergic reaction).
- •
Because transitional cell carcinoma is so uncommon in young, nonsmoking patients, MR urography or ultrasound may serve as an appropriate screening study following a negative renal colic CT examination.
Introduction
Magnetic resonance urography (MRU) and computed tomography urography (CTU) are useful tools that provide a comprehensive assessment of the renal parenchyma, renal collecting systems, and ureters. Cystoscopy remains the reference standard for bladder evaluation, which is not discussed here. This article reviews the indications and optimal technique for MRU and CTU. The appearance of benign and malignant disease is also reviewed.
Background
CTU has become the most frequently used screening test for upper urinary tract tumors because of its widespread availability, short imaging time, and high spatial resolution. It is the reference standard for evaluation of the upper urinary tract. Drawbacks include use of iodinated contrast, which can exacerbate renal insufficiency and cause allergic-type reactions. CTU also imparts a relatively high level of ionizing radiation exposure, which is undesirable in the setting of pregnancy, pediatric patients, and frequent imaging.
MRU has the benefits of improved soft tissue resolution, absence of ionizing radiation, and a greater safety profile for intravenous contrast agents due in part to lower doses relative to CTU. Disadvantages include long scan times, more limited availability, and difficulties due to imaging artifacts and respiratory motion. Diuretics are important adjuncts for the examination. Rarely, these may be associated with allergic reactions. The American College of Radiology (ACR) Contrast Manual version 10.3 reports screening for renal impairment is now optional when using a macrocyclic agent such as Gadavist (gadobutrol; Bayer, Leverkusen, Germany) or Dotarem (gadoterate meglumine; Guerbet, Villepinte, France).
Imaging techniques
Magnetic Resonance Urography
MRU can be performed on 1.5-T or 3.0-T systems using an identical protocol with similar results. The patient is placed in supine position with arms raised above the head if possible. External torso phased array coils are placed over the patient to cover the kidneys and bladder in a single field of view.
Adequate distention of the urinary tract is essential for evaluation of nonobstructed collecting systems. If contrast is to be administered, gadolinium chelates can cause paradoxic low signal intensity due to the T2* effect overcoming the effect of T1 shortening. This can be minimized by administration of diuretics, normal saline, or both. At our institution, 10 mg furosemide is administered intravenously 5 minutes before administration of contrast agent; 250 mL intravenous (IV) saline can be used in the setting of diuretic allergy or dehydration. If a bladder catheter is present, it is clamped before diuretic or fluid administration.
Sample protocols from our institution, including both contrast-enhanced and noncontrast MRU, are given in Tables 1 and 2 . Both protocols include axial and coronal single-shot fast spin-echo (SSFSE) images and axial T1 in-phase and opposed-phase T1-weighted images of the abdomen, and an axial T2-weighted fast spin-echo sequence of the bladder. In noncontrast technique, additional thick slab and 3-dimensional (3D) or thin-section highly T2-sensitive MR cholangiopancreatography (MRCP)-like sequences are acquired ( Fig. 1 ). If contrast is used, 0.1 mmol/kg is administered at 2 mL/s and 3D fat-saturated T1-weighted spoiled gradient echo (SPGR) images are obtained at 40 seconds, 90 seconds, and 10 minutes, usually in the coronal projection. Fat saturation can be achieved with spectral fat suppression, or Dixon technique, the latter using the water-only reconstructed images. From the delayed sequence, an MIP (maximum intensity projection) is obtained, generating an IV pyelogram (IVP)-like image ( Fig. 2 ). Subtraction images also can be obtained. If the technologist notices a region with suboptimal imaging, additional images can be obtained. This is an advantage compared with CTU, in which each acquisition results in additional radiation exposure.
| Coil: External Phased Array Torso | |||||||
|---|---|---|---|---|---|---|---|
| Plane | Sequence | Size/Gap (2D) or Slice/Interval (3D) in mm | Region/Comment | Matrix | FOV cm | TR ms | TE ms |
| COR | 2D T2 SSFSE | 6/1 | Abdomen and Pelvis (K, U, B) | 288 × 248 | 40 × 40 | 507 | 80 |
| AX | 2D T2 FSE | 6/1 | Pelvis | 265 × 217 | 21 × 21 | 4819 | 120 |
| AX | 2D T1 IP/OP | 6/1 | Abdomen | 220 × 217 | 33 × 40 | 91 | 2.3/4.6 |
| AX | 2D T2 SSFSE | 6/1 | Abdomen | 236 × 239 | 26 × 26 | 1299 | 80 |
| SAG | 3D T1 mDIXON Pre | 3/1.5 | Left Kidney Single Phase | 200 × 210 | 30 × 31 | 5.9 | 1.8/4.0 |
| SAG | 3D T1 mDIXON Pre | 3/1.5 | Right Kidney Single Phase | 200 × 210 | 30 × 31 | 5.9 | 1.8/4 |
| CLAMP BLADDER CATHETER IF PRESENT 10 mg IV furosemide given slowly over 1 min prior to dynamic contrast enhanced sequences | |||||||
| COR | 3D T1 mDIXON Dynamic | 3/1.5 | Abdomen and Pelvis Pre 40 sec 90 sec 10 min | 176 × 173 | 35 × 35 | 5.8 | 1.80/4 |
| SAG | 3D T1 mDIXON Post | 3/1.5 | Left Kidney Single phase | 200 × 210 | 30 × 31 | 5.9 | 1.8/4 |
| SAG | 3D T1 mDIXON Post | 3/1.5 | Right Kidney Single phase | 200 × 210 | 30 × 31 | 5.9 | 1.8/4 |
| AX | 3D T1 mDIXON Top | 3/1.5 | Single Phase Abdomen | 176 × 160 | 35 × 31 | 5.9 | 1.8/4 |
| AX | 3D T1 mDIXON Bottom | 3/1.5 | Single Phase Pelvis | 176 × 160 | 35 × 31 | 5.9 | 1.8/4 |
| COR | 3D T1 SPGR | 3/1.5 | Flip Angle = 40° 10 min delay | 176 × 160 | 35 × 35 | 20 | 4.6 |
| Coil: External Phased Array Torso | |||||||
|---|---|---|---|---|---|---|---|
| Plane | Sequence | Size/Gap (2D) or Slice/Interval (3D) in mm | Region/Comment | Matrix | FOV cm | TR ms | TE ms |
| CLAMP BLADDER CATHETER IF PRESENT 10 mg IV furosemide given slowly over 1 min at beginning of examination | |||||||
| COR | 2D T2 SSFSE | 6/1 | Abdomen and Pelvis (K, U, B) | 288 × 248 | 40 × 40 | 507 | 80 |
| SAG | 3D T2 FSE | 4/2 | Pelvis | 200 × 145 | 20 × 20 | 2000 | 200 |
| AX | 2D T2 SSFSE | 5/1 | Abdomen | 236 × 239 | 26 × 26 | 1299 | 80 |
| AX | 2D T2 SSFSE | 5/1 | Pelvis | 265 × 217 | 21 × 21 | 4819 | 120 |
| AX | 3D T1 mDIXON Top | 3/1.5 | Abdomen | 176 × 160 | 35 × 31 | 5.9 | 1.8/4 |
| AX | 3D T1 mDIXON Bottom | 2/1 | Pelvis | 176 × 160 | 35 × 31 | 5.9 | 1.8/4 |
| SAG | 2D T2 fs SSFSE | 4/0.4 | Left Kidney | 200 × 210 | 30 × 31 | 5.9 | 1.8/4 |
| SAG | 2D T2 fs SSFSE | 4/0.4 | Right Kidney | 200 × 210 | 30 × 31 | 5.9 | 1.8/4.0 |
| AX | DWI | 5/0.5 | Abdomen and Pelvis | 104 × 121 | 31 × 37 | 1411 | 61 |
| COR | 3D T1 mDIXON | 3/1.5 | Abdomen and Pelvis | 176 × 173 | 35 × 35 | 5.8 | 1.80/4 |
| RAD | 2D T2 SSFSE Thick Slab | 40/0 | MRCP-like | 320 × 256 | 30 × 30 | 8000 | 800 |
| COR | 3D T2 SSFSE Thin | 2/1 | MRCP-like | 260 × 260 | 26 × 26 | 1024 | 600 |


Computed Tomography Urography
Most CTU protocols consist of 3 phases (unenhanced, nephrographic, and excretory). Unenhanced images are obtained of the kidneys, ureters, and bladder (3-mm slice thickness, 3-mm gap); 100 mL iodinated contrast (Omnipaque 350 [iohexol], GE Healthcare Inc, Marlborough, MA or Isovue 370 [iopamidol], Bracco, Monroe Township, NJ) is then administered at 3 mL/s followed by 150 mL of 0.9 normal saline at the same rate via power injector. Nephrographic-phase images of the entire abdomen and pelvis are obtained at 100-second delay. Slice thickness is typically 3 mm, but the gap can be variable. Ten-minute delayed-phase images are obtained in the axial plane (3-mm slice thickness, 3-mm gap) of the kidneys, ureters, and bladder. Coronal and sagittal multiplanar reconstruction (MPR) images (2-mm thick, 2-mm gap) of all phases are obtained. Review of all planes is essential for detection of small urothelial lesions, particularly on the delayed-phase images. Furthermore, the delayed-phase images must be evaluated with appropriate window and level settings to detect subtle abnormalities that can be obscured by dense contrast. To reduce radiation dose, the split bolus technique optimizes contrast timing to obtain both nephrographic and excretory phases simultaneously in a single acquisition. Iterative reconstruction, low dose unenhanced scans, and dual energy strategies are other useful or promising techniques for dose reduction.
Indications
Indications for MRU and CTU include hematuria (both microscopic and macroscopic), urinary obstruction, evaluation for renal and urothelial neoplasms, and characterization of congenital abnormalities. As renal stones are the most common cause of hematuria, ultrasound or noncontrast CT are often the first tests obtained.
Both the ACR and the American Urologic Association (AUA) have generated recommendations for the evaluation of hematuria in adults, albeit with several important differences. Specifically, the AUA guidelines do not address radiation dose in young patients without significant risk factors or the cumulative radiation exposure of serial examinations. Summaries of the ACR and AUA recommendations are given in Boxes 1 and 2 .
- •
Most adults with gross or persistent microscopic hematuria require urinary tract imaging. Urinary tract imaging is NOT recommended in the following subgroups: young female patients with cystitis, hematuria that resolves completely, recent infection or viral illness, vigorous exercise, or recent urologic procedures including catheterization.
- •
When imaging of the urinary tract is warranted, computed tomography urography (CTU) is the preferred examination. CTU has largely replaced the traditional intravenous pyelogram for this indication.
- •
MR imaging is an excellent technique to evaluate for renal masses and complex cysts. If there are contraindications to iodinated contrast, MR urography (MRU) is a good substitute examination, but is inferior to CTU in detection of stones and remains under investigation for detection of urothelial lesions.
- •
Ultrasound is the first-line modality in patients with suspected renal parenchymal disease in setting of microscopic hematuria.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree