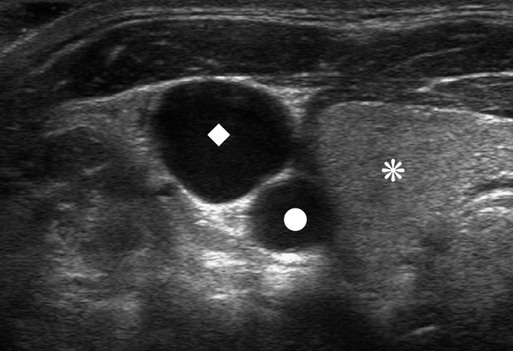
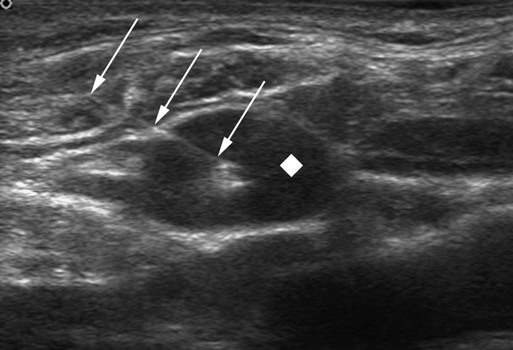
The history of CVCs dates back to 1733, where an English clergyman, Stephen Hales, inserted a glass tube into the jugular vein of a horse to measure pressure. The first prolonged use of catheters for central venous infusion was reported by B.J. Duffy in 1949, reporting on 72 catheters.1 The catheter we use most often today was created at the University of Washington by a team including Belding Scribner, John Broviac, and Robert Hickman in 1973.2 The basic design of the catheter has not changed, although new technologies have allowed different brands to distinguish themselves. Over the past years, valve technology was developed to reduce thrombosis and infection and eliminate the need for heparin packing. Recently, with the advent of faster computed tomography (CT) scanning, power injectibility has been introduced. CT has become an irreplaceable technology for diagnosing all types of diseases. Newer protocols rely on rapid introduction of a bolus of contrast material for CT angiography and evaluation of tissue perfusion. Many patients with chronic disease undergo regularly scheduled CT scans to evaluate the effectiveness of treatment, and these same patients have some form of central venous access for that treatment. Rather than start a peripheral intravenous line each time the patient needs a CT scan, it would be desirable to use the patient’s existing access. This has been accomplished with newer power-injectable cuffed tunneled catheters. The use of polyurethane for the construction of the catheter has allowed larger inner lumens and faster flow rates. The Power Hickman (Bard Access Systems [Fig 119-1]) was the first such catheter. It is capable of injection rates up to 5 mL/s, which is clearly marked on the catheter’s hub. In addition, the catheter has a distinct purple color that has become a universal signal of power injectibility, present on PICC lines and ports from Bard as well as other manufacturers. Although there are many studies3–6 that have safely shown the use of regular catheters for power injection, these were done off-label, and the readily available power injection safe catheters today render this practice obsolete. The right internal jugular (RIJ) vein is the preferred vessel for entry when all other variables are equal. The modern proceduralist will always use ultrasound to guide venous access. When ultrasound is used, the RIJ has been shown to have the lowest rate of complications, including thrombosis, arterial puncture, pneumothorax, and catheter malposition.7–13 Although some articles have recently settled on the subclavian vein (SCV) as the preferred entry site, this recommendation is for temporary catheters, not tunneled ones. The Kidney Disease Outcomes Quality Initiative (K/DOQI)14 clearly states that the RIJ is the primary site for dialysis catheters and that tunneled infusion catheters should follow suit. In a retrospective review of subclavian versus IJ tunneled catheters by Trerotola et al. in 2000,9 there was a 13% incidence of clinically significant central thrombosis when catheters were placed in the SCV (with fluoroscopic guidance) versus 3% when placed in the IJ (with ultrasound guidance). Of note, they did not identify a higher risk for infection in the IJ than in the subclavian site. When the RIJ is not available, the next vein of choice is somewhat controversial. Most interventionalists will proceed to the left internal jugular (LIJ) or resort to the SCV. However, a recent article by Cho et al. proposed that the right external jugular vein is the preferred second choice. They demonstrated an acceptable success rate and a low complication rate to reach their conclusion. This makes sense because of the tortuous route through the left brachiocephalic vein from an LIJ approach. The final step in patient preparation is a preliminary ultrasound scan of the proposed catheter site before the patient is sterilely prepared. The technique for prescanning includes identification of the jugular vein and its relationship to the carotid artery (Fig. 119-2). This is done in the transverse plane and carried down to below the clavicle until the vein cannot be visualized. Test the compressibility of the vein to identify it and exclude acute thrombosis. Note the location of the subclavian artery if it is present and its relationship to the IJ. Finally, look for valves in the jugular bulb. If these valves are moving back and forth with respiration, there is direct communication with the right atrium (RA), which helps assure the operator there is not a significant stenosis in the superior vena cava (SVC). The technique for RIJ access (Fig. 119-3) is as follows. Position a linear 7- to 12-MHz transducer that has been placed in a sterile cover parallel to and touching the clavicle. Identify the RIJ, and palpate the sternal head of the sternocleidomastoid muscle. The puncture site should be just lateral to this muscle, which allows superolateral entry into the RIJ. Use liberal lidocaine with epinephrine and create a wheal. Make the incision appropriate for the catheter size. Use an angled hemostat to dissect the tissues down to the vein and in the direction of the tunnel. With the vein seen in transverse orientation and the needle in longitudinal orientation, advance the needle into the vein until it is seen tenting the wall. Make sure to angle the transducer to visualize the entire subcutaneous course of the needle. When tenting the wall, slowly advance until the wall release and the tip of the needle in the vein are seen (Fig. 119-4). We use a 21-gauge needle with a 0.018-inch wire for most accesses. If resistance is encountered while advancing the wire from the needle tip, pull the wire back until it is in the needle and then pull the needle back. Readvance the wire until no resistance is met. This maneuver can be repeated until the needle is out of the skin. If at any time resistance is encountered when pulling the wire back into the needle, stop and pull the needle and wire out as a unit. Mandrel-type wire tips can shear off and embolize to the lungs. Once the wire is safely in the vein, advance it several centimeters into the RA while being careful to listen for ectopy, remove the needle, and place a transitional dilator. I prefer the Cope Access Set (Cook Medical, Bloomington, Ind.), which includes a 6.3F dilator with a metal inner stiffener, a 0.018-inch stainless steel Cope mandrel wire, and a 0.035-inch Rosen wire.
Tunneled Central Venous Catheters
Equipment
Technique
Anatomy and Approach
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine