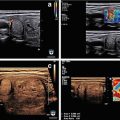
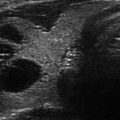
Figure 9.1
Superior parathyroid adenoma seen in longitudinal view. Indentation sign. (a) Upper PA, craniocaudal view, note indentation. (b) Upper PA, note indentation sign, and long pencil shape. (c) Small superior adenoma
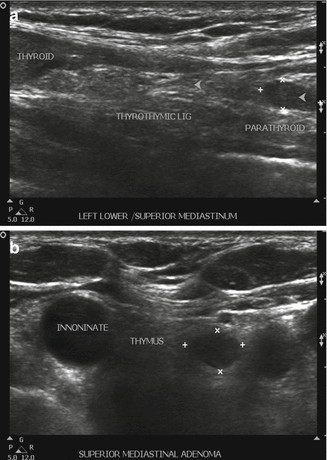
Figure 9.2
(a, b) PA located in the proximal portion of thyrothymic ligament and within the thymus

Figure 9.3
Superior parathyroid adenoma seen in transverse view
US Findings of Parathyroid Adenomas
Extrathyroidal and posterior location
Homogenous hypoechoic texture
Echogenic line of separation—this sign is absent in intrathyroidal adenomas
Distinct vascular pedicle or polar vessel on Doppler interrogation
Variable shapes conforming to available space within anatomical planes
Indentation sign—superior or inferior glands that are abutting the posterior capsule
One postmortem series revealed the presence of four glands in 91% of the subjects, three glands in 5%, and five glands in 4% [6]. Supernumerary glands are rare and found in less than 5% of individuals [10]. The locations of parathyroid glands vary widely due to their embryonic origination from the third and fourth pharyngeal pouches with eventual migration to the lower neck. The superior parathyroid glands develop in the fourth pharyngeal pouch and migrate caudally along with the ultimobranchial bodies, which give rise to parafollicular cells—C cells of the thyroid gland. The superior pair is commonly located posterior to the thyroid lobes along the mid to upper two thirds of the thyroid lobes. The superior parathyroid glands are relatively constant in their location. Anomalous locations of the superior glands include the posterior pharyngeal region and the tracheaesophageal groove; however, they are also more likely to be located within the upper and posterior aspect of the thyroid lobes due to their unique developmental relationship to the C cells. The third branchial pouches give rise to the inferior pair of parathyroid glands and the thymus; together they migrate to the lower neck. Forty-four percent of inferior pair are located within 1 cm of the inferior pole of the thyroid gland, 17% are in close proximity to the inferior margin of the thyroid gland, 26% are found close to the superior portion of the thymus along the thyrothymic ligament (Fig. 9.2), and 2% are found within the thymus [8–10]. The unusual locations of the inferior parathyroid glands include the carotid bifurcation, the carotid sheath, intrathyroidal, and retropharyngeal sites. The reported frequency of ectopic parathyroid glands is quite variable ranging from 5 to 39.3%. This variability is due to the lack of criteria and agreement between investigators as to which locations should be considered as ectopic. A Greek autopsy series involving 942 autopsies revealed 5% of cadavers had supernumerary glands, 2% were intrathyroidal, and 6% of the glands were mediastinal in location. Most glands were located within the immediate vicinity of the thyroid gland [11]. In contrast, in a surgical series involving four gland surgeries for secondary hyperparathyroidism due to chronic renal failure, 39.3% of the glands were reported by the authors as “ectopic” in location [12]. Nevertheless, the variable location of the parathyroid glands underscores the importance of localization to ensure the success of minimally invasive parathyroid surgery.
Epidemiology of Primary Hyperparathyrodism
The predominant clinical phenotype of PHPT in the developed nations is the fortuitous discovery of asymptomatic individuals with normal or mild hypercalcemia undergoing yearly physical examinations. The apparent increase in the incidence of PHPT in the 1970s was traced to the development and wide availability of multichannel analyzers [2]. In a study from the USA, involving 3.5 million people, the incidence of PHPT varied from 34 to 124/100,000 person years. There is an age-related increase in incidence with the highest prevalence in Blacks followed by Whites. Latino and Asian minorities had the lowest incidence. PHPT is also threefold more common in women than in men [13]. The volume of surgeries performed for PHPT has increased 177% in California during 1999–2008 [14].
The typical end-organ damage encountered in PHPT patients is due to bone loss at the cortical bone sites and renal stones. Trabecular bone (spine) is relatively preserved at the expense of cortical bone sites such as the femoral neck and ultra-distal radius [15].
Due to the online heightened awareness among patients and doctors and because of “proactive” PTH testing in normocalcemic subjects, there is an emerging trend in the diagnosis of “eucalcemic” hyperparathyroidism. Selective administration of surgery following careful evaluation of end-organ damage in such patients is reasonable until randomized studies are conducted.
Localization Studies
Ultrasound evaluation of the parathyroid glands should not be performed to diagnose PHPT. Its use should be reserved strictly for localization purposes. Appropriate biochemical testing should be conducted and surgical indication(s) established in each patient prior to proceeding to any type of localization studies.
The two most widely used studies to locate the abnormal parathyroid gland(s) are Tc99MIBI (functional study) and ultrasonography (anatomical study). The respective localization techniques have their strengths and weaknesses and are equally efficacious [16]. Most isotope-based studies “lateralize” the lesion, whereas US studies provide precise localization information. To a practicing endocrinologist, the use of ultrasonography to study a patient with suspected parathyroid adenoma poses several advantages. The most important of these are the proven safety, ease of availability of ultrasound equipment, the lack of ionizing irradiation, short duration of the study, and the potential cost savings. The limitations of parathyroid localization using ultrasonography include operator variability and experience.
The Technique of Performing Ultrasonography for PHPT
Proper positioning of the patient is crucial to the su ccessful visualization of enlarged parathyroid gland(s). The patient should lie flat on a firm table with one or two pillows placed underneath the posterior upper torso and the shoulders to enable full extension of the neck. The head should be supported with a folded towel to enhance patient comfort. Patients with cervical spine diseases such as ankylosing spondylosis may have very limited neck extension making it impossible to conduct an adequate study.
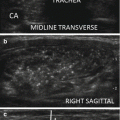
The linear transducer (3–5 cm) is applied to the anterior neck along with appropriate amount of coupling gel, and the thyroid gland is located. The structures of the neck should be carefully studied in multiple axes and at different levels of the neck. Most clinicians use multifrequency transducers (5–15 MHz) to study the thyroid gland. Parathyroid ultrasonography does not require different equipment. The lower frequency settings are more effective at visualizing the deeper portions of the neck. The most common sites of location, such as the posterior margin of the thyroid capsule and the regions caudal to the thyroid lobes, are inspected first looking for lesions with features of enlarged parathyroid gland. Due to the inherent mobility of parathyroid adenomas, the tumor may not be visualized readily, particularly the superior adenomas located within the tracheaesophageal groove or intrathoracic inferior gland adenomas. Asking patients to cough, strain, turn the head from side to side, or take deep breaths in and out can enable visualization of a mobile adenoma (Fig. 9.4).
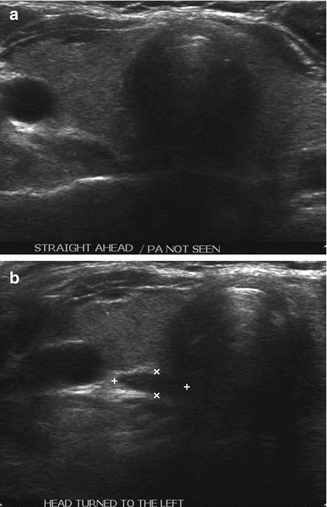

Figure 9.4
(a, b) Mobile right upper TE groove adenoma
Ultrasound Evaluation in MEN Syndromes and Chronic Renal Failure Patients
Multiple Endocrine Neoplasia
Primary hyperparathyroi dism occurs in MEN 1 and MEN 2a syndromes. In these syndromes, multigland disease is very common. All gland inspection is required during surgery even if localization studies reveal unilateral abnormality; therefore, US or Tc99MIBI localization is of little value. However, there is a role for performing US evaluation in MEN subjects who have had unsuccessful surgeries.
Renal Failure
In subjects with chronic renal failure , all glands are affected to a variable degree. Surgical intervention should involve inspection of all parathyroid glands; therefore, localization is of limited value. US evaluation is a valuable tool to perform ETOH ablation in ESRD patients who are unable to undergo surgery due to comorbidities. Renal patients also present an excellent opportunity to practice parathyroid ultrasonography.
Ultrasound Features of the Parathyroid Adenomas
Normal or non-enlarged parathyroid glands cannot be visualized with present-day US equipment. In eucalcemic or mildly hypercalcemic subjects, it is possible to have a diminutive adenoma in the typical sites of location which elude US detection. Ultrasound evaluation cannot be used to diagnose PHPT; it is strictly a localization tool. The following are the distinct ultrasonographic features of parathyroid adenomas.
Extrathyroidal Location and Indentation Sign
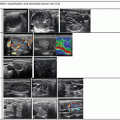
The vast majority of parathyroid adenomas are located outside the posterior capsule of the thyroid gland [10–12]. These lesions are located in close relation to the posterior capsule of the thyroid gland [17]. The visualization of a lesion along the posterior aspect of thyroid gland in the clinical context of hypercalcemia makes the possibility of a parathyroid gland very likely. It is quite common to see an indentation made by the parathyroid adenoma on the posterior capsule of the thyroid gland: “the indentation sign.” There is also a noticeable echogenic line observed, separating the two glands, which represents the fibro-fatty capsule. A parathyroid adenoma is embedded within the thyroid gland in about 2–5% of cases [18, 19] when it is indistinguishable in appearance from a thyroid nodule. The incidence of intraglandular location was higher in patients with multigland disease in one series: 3% in patients with uniglandular disease versus 15% in those with hyperplasia [13] (Figs. 9.3, 9.5, and 9.6).
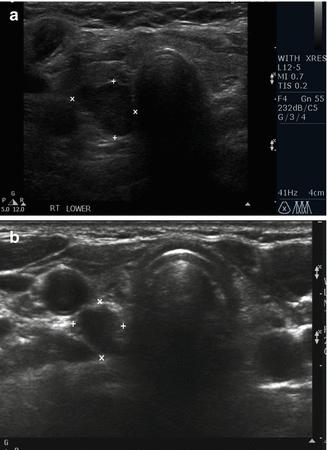


Figure 9.5
(a, b) Inferior parathyroid adenoma in transverse view

Figure 9.6
Inferior parathyroid adenoma seen in longitudinal view. (a) Inferior, intrathyroidal, craniocaudal. (b) Inferior PA, craniocaudal, pencil-shaped lesion. (c) Inferior paravertebral posteriorly located adenoma
Homogenous Hypoechoic Texture
This is a distinguishing imaging characteristic of parathyroid adenomas. The enlarged parathyroid glands are homogeneously hypoechoic echotexture in relation to the thyroid gland [20].
Vascular Pedicle and Blood Flow
The presence of an independent artery (polar artery) feeding an adenoma was found in 83% of parathyroid adenomas [21]. Other patterns of blood flow such as the “vascular arc” and diffuse flow within the adenoma have also been described [22] (Fig. 9.7).
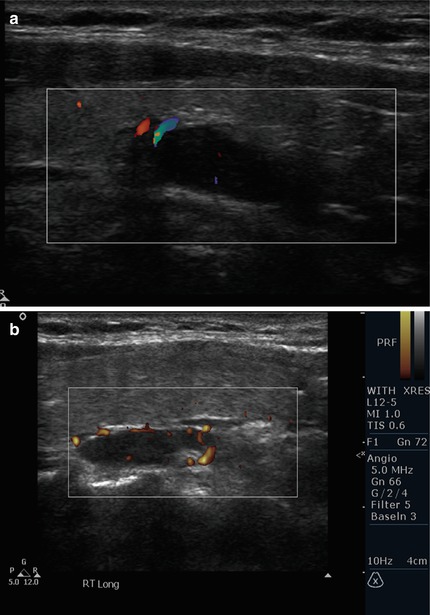



Figure 9.7
(a) Polar vascular pedicle color Doppler. (b) Polar vessels depicted by power Doppler. (c) Arc pattern of blood flow. (d) Diffuse blood flow seen within adenoma
Variable Shapes
Parathyroid adenomas dev elop along the fascial planes and conform to the anatomical pressures of surrounding structures. Therefore, considerable variations in shapes can be observed. Oval, sausage shaped, pencil shaped, triangular, or oblong shapes can all be observed.
Lack of US Visualization of Parathyroid Lesion(s) in Hypercalcemic Subjects
In the hands of experienced operator s, parathyroid ultrasonography has high level of sensitivity and specificity. Despite the best of efforts, in about 10–20% of the subjects, adenomas are not visualized. The likely cause includes posteriorly located lesions, retropharyngeal superior adenomas, intrathoracic location, and parathyroid hyperplasia. In one study, posteriorly located parathyroid adenomas were observed in 58% of subjects with negative US and Tc99MIBI scans [23].
Parathyroid Incidentaloma
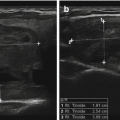
Subclinical parath yroid tumors can be discovered incidentally during neck ultrasonography (Figs. 9.8 and 9.9). The frequency of observing these incidental tumors is rare [24, 25]. Fine needle aspiration with PTH estimation in syringe washings is a reliable way to identify these lesions as parathyroid tumors.
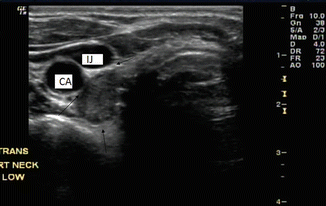

Figure 9.8




Adenoma within the carotid sheath
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree