Fig. 25.1
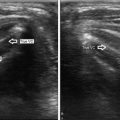
Early planar Sestamibi scan in a patient with Graves’ disease treated with RAI. Notice absence of uptake in the left thyroid lobe and positive uptake in the right thyroid and lower cervical parathyroid

Fig. 25.2
Ultrasound sagittal view of the right lobe confirms the presence of multiple thyroid nodules (red arrow) and a right inferior parathyroid (white arrow) in a patient with Graves’ who had prior RAI ablation

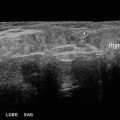
Fig. 25.3
Ultrasound in transverse view showing an arc of vascularity around the right inferior parathyroid in a patient with Graves’ treated with RAI ablation

Fig. 25.4
Planar Sestamibi scan showing a right-sided likely superior parathyroid

Fig. 25.5
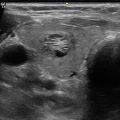
Transverse view ultrasound indicating that the parathyroid gland, which was identified on Sestamibi scan, is intra-thyroidal (arrow)
25.3.2 US to Localize the Parathyroid and Help with Operative Planning
When US is performed by an experienced sonographer it can accurately identify the parathyroid abnormality in 76–89 % of cases and help the surgeon direct the exploration [13–19]. A parathyroid that is behind the mid or upper portion of the thyroid lobe on ultrasound is usually a superior gland and it will likely be in a posterior location at exploration. An enlarged parathyroid that is near the lower pole of the thyroid or near the clavicle is most likely an inferior gland and the surgeon should look in a more anterior location near the lower thyroid pole, the thyro-thymic ligament, or the thymus (see the next section for interpretation of US images). A “negative” US can inform the surgeon on “what is not there.” For instance, with a “negative” US, the surgeon can surmise that the adenoma may be in a posteriorly located para-esophageal/tracheoesophageal position, which is not easily imaged by US, and direct the exploration to such area first. In this patient the MIBI could show a “low cervical adenoma” confirming that this is a superior gland that has moved to the low neck by growth (Fig. 25.6). Furthermore, the sonographer should not accept a “negative” US unless the upper neck, the carotid sheath, and the area just under the clavicle and sternal notch have been carefully imaged (see below).


Fig. 25.6
Planar delayed view of Sestamibi scan showing a lower cervical parathyroid adenoma (arrow). Because the ultrasound was “negative” (not shown) the surgeon should suspect this to be a superior gland deep in the tracheoesophageal groove
In patients with prior thyroid operations or after ablative RAI (Figs. 25.1, 25.2, and 25.3) US is critical to evaluate whether thyroid tissue is still present in the neck and to correlate with MIBI scan (if one was performed). The radiologist reading the MIBI scan and without clinical history may assume incorrectly that what is imaged in the para-tracheal space is thyroid when in fact it is parathyroid tissue in the setting of ablated, surgically removed, or agenesis of the thyroid lobe (Fig. 25.7, Video 25.1).


Fig. 25.7
Planar early phase Sestamibi showing an “atrophic” left lobe (arrow)
A contemporary meta-analysis evaluated the accuracy of common preoperative localization techniques in patients with primary hyperparathyroidism against the gold standard of intraoperative visualization and histology. The pooled sensitivity and positive predictive value of US was 76.1 % (57–89 %) and 93.2 % (85–100 %), respectively [20]. Ruda et al. reviewed the literature from 1995 to 2003 and reported US sensitivity of 79 % in localizing abnormal parathyroid glands [21]. It is important to know that the results of US are operator dependent and can be negatively influenced by the presence of thyroid nodular disease, increased body mass index, presence of multiglandular/ectopic parathyroid disease, and small parathyroid size [22]. Posterior thyroid nodules and cysts can be mistaken for a parathyroid adenoma and visa-versa. US is less sensitive when parathyroids are located behind the trachea, esophagus, in the superior mediastinum and in patients with thyroiditis who may have extensive reactive adenopathy.
25.3.3 Cost-Effectiveness of Ultrasound
Wang et al. reported on a cost-utility analysis to optimize preoperative imaging for HPT [23]. A decision tree was constructed to determine the incremental cost-utility ratio of five localization strategies: (1) US; (2) MIBI with single photon emission tomography (SPECT); (3) 4DCT; (4) MIBI-SPECT and US; and (5) MIBI-SPECT and US ± 4DCT (4DCT added when MIBI and US were discordant). The authors concluded that US is the least expensive imaging modality. Yet, the most cost-effective strategy involved the use of US combined with MIBI-SPECT and if needed 4DCT. This strategy costs less and accrued more “utility” (a measure of quality of life) mostly because BE was avoided, hence decreasing the overall cost of parathyroidectomy . Interestingly, utilizing MIBI-SPECT as the sole localization study was the least cost-effective strategy.
In another economic analysis of preoperative localization strategies for HPT , Lubitz and colleagues constructed a decision-analytic model to evaluate eight different localization strategies [24]. The authors concluded that US followed by 4DCT when the US results were indeterminate, was the least costly strategy. Differences in cost were largely based on improved sensitivity for detecting single-gland disease and, therefore, on the proportion of patients able to undergo minimally invasive focused procedures with shorter operating time and same-day discharge.
Both studies mentioned above concluded that the cost of BE without localization was always higher, even when the sensitivity of localization studies was lowest (i.e., localization studies were least useful). Most cost-effectiveness studies assume that the majority of patients undergoing BE will require longer operating times and overnight stays in the hospital, therefore driving the costs of parathyroidectomy higher. Currently, a growing number of high-volume parathyroid surgeons who perform BE via small incisions will discharge their patients the same day, possibly neutralizing the cost of such approach when compared to minimally invasive procedures [25, 26].
The authors use surgeon-performed US (SUS) in all patients with HPT who are scheduled for parathyroidectomy . When a clear parathyroid adenoma is localized by SUS, patients are explored using US as the only localization study [14, 27]. However, the authors always use IPM to avoid missing multiple gland disease. Recently, Untch et al. confirmed previous findings that SUS has the same sensitivity as MIBI scans and can be used as the only localization study resulting in excellent operative success when parathyroidectomy is guided by IPM [17].
25.4 Parathyroid Embryology and Anatomy as It Relates to Parathyroid Ultrasound Interpretation
Ideally, the sonographer and the operating surgeon should have a thorough understanding of parathyroid embryology and anatomy. Most patients will have four parathyroid glands (two superior and two inferior), although <4 glands and supernumerary glands can be encountered in up to 3–13 % of cases, respectively [28, 29]. Understanding the parathyroid glands eutopic and ectopic locations is of utmost importance in the interpretation of imaging studies as it relates to the surgical procedure. The surgeon should always review all the radiologic images when planning the operative approach never relying on the radiology report alone. The superior glands are derived from the fourth branchial pouch along with the lateral lobes of the thyroid. The inferior glands arise from the third pouch along with the thymus (further discussion below). Superior and inferior glands are typically symmetric to each other (80 % and 70 % of the time respectively).
25.4.1 Typical Ultrasound Appearance and Location of Parathyroid Adenomas
The typical US appearance and location of an inferior parathyroid adenoma is depicted in Figs. 25.8 and 25.9 and Video 25.2. On gray-scale imaging, parathyroid adenomas are predominantly solid lesions with well-defined margins. Internal echo texture is nearly always homogenous and hypoechoic when compared to the thyroid (Table 25.1). A parathyroid is usually separated from the thyroid by a well-defined echogenic tissue plane [30]. Enlarged parathyroids are almost always >1 cm, oval but can also be kidney shaped, bilobed, or multi-lobulated. On color Doppler sonography, parathyroid adenomas are very vascular. Color Doppler usually shows a peripheral vascular arc arising from the inferior thyroid artery and encircling the gland 90–270° (Fig. 25.3). This feature may help distinguish a parathyroid from a lymph node. The polar artery usually arborizes around the parathyroid vs. a lymph node will have a more hilar vascular flow (Video 25.3). Atypical parathyroids may be cystic (Figs. 25.10 and 25.11), heterogenous, echogenic, have a dual concentric sign and/or calcified (Fig. 25.12) [31–33].






Fig. 25.8
Transverse ultrasound view of a typical wedge-shaped inferior parathyroid adenoma (arrow)

Fig. 25.9
Longitudinal ultrasound view of a typical inferior parathyroid adenoma (arrow)
Table 25.1
Typical and atypical ultrasound characteristics of abnormal parathyroids
Typical | Atypical |
|---|---|
Solid | Cystic areas |
Homogenous | Heterogenous |
Smooth well defined borders | Infiltrative or irregular borders |
Oblong, tear drop, or oval shaped | Lobulated |
Not calcified | Calcified |
Polar artery on Doppler | Highly vascular on Doppler |
Single | Multiple |
Located along the margins of the thyroid gland | Ectopic locations: retro-esophagus, high in the neck above the thyroid, in the thymic tongue, deep in the mediastinum, in the thyroid |

Fig. 25.10
Transverse ultrasound view of a cystic and heterogenous left parathyroid adenoma

Fig. 25.11
Longitudinal ultrasound view of a cystic and heterogenous left parathyroid adenoma

Fig. 25.12
Longitudinal ultrasound view of a calcified left superior parathyroid in a renal failure patient (arrow on calcium)
The three most common locations for the inferior parathyroid can be easily imaged by US (see below). Sonographers should always scan posterior to the clavicle by angling the US probe under it and asking the patient to swallow. This maneuver is helpful in localization of thymic or thoracic inlet parathyroid glands. Such glands can be common in patients with secondary hyperparathyroidism due to renal failure (Fig. 25.13a, b; Video 25.3). When US fails to reveal an abnormal parathyroid in its eutopic location, the lateral high neck, particularly the area medial to the carotid artery (Level II lymph node basin/carotid bulb area) should be carefully scanned looking for a rare ectopic and/or undescended gland.


Fig. 25.13
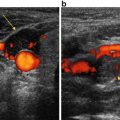
(a) Longitudinal ultrasound view of a heterogenous thyro-thymic parathyroid in a patient with renal failure. Note that the overlying strap muscles are seen to diverge toward the right side of the panel (caudad on the patient)—this is a clue that the longitudinal image captured is from a location close the clavicles. (b) Dual phase planar sestamibi on the same patient confirming the thyro-thymic left-sided parathyroid
The location of the superior parathyroid is less variable, yet its posterior position can be challenging to visualize with US, particularly in obese patients, male patients, those with goiters, or in the presence of thyroiditis (Fig. 25.14).


Fig. 25.14
Transverse ultrasound view of a typical hypoechoic oblong superior right-sided parathyroid (arrow)
25.4.2 Superior Parathyroid Anatomic and Imaging Location
The superior parathyroids are usually located in a posterior plane when compared to the inferior glands. The possible location of the superior parathyroid is posterior to the mid portion of the superior thyroid lobe near the cricothyroid junction (>90 %) posterior to the mid thyroid lobe (4 %) (Figs. 25.15 and 25.16), superior to the thyroid lobe (3 %) (Fig. 25.17a–c), in the retropharyngeal/retroesophageal location (1 %), or intra-thyroidal (0.2 %) [28, 29, 34]. A superior gland that has “fallen down” due to growth and gravity into the tracheoesophageal groove or para-esophageal area can be imaged in a relatively low cervical location [35] (Fig. 25.18). The inexperienced radiologist or surgeon may interpret this as an inferior gland when in fact it is a superior gland that has grown enough to migrate low in the neck [36]. In effect, one of the most common locations of a missed parathyroid during a prior failed parathyroidectomy is a superior gland in the posterior and low paraesophageal area [37]. The posterior location of the superior gland can, therefore, be confirmed on ultrasound (Fig. 25.18, Video 25.4), 4DCT, SPECT/CT MIBI images, or the oblique images of a planar dual phase MIBI scan. Similarly, a retropharyngeal/retroesophageal superior gland may not be apparent on US while 4DCT and MIBI with SPECT or MIBI SPECT/CT are more suitable to image such posterior/deep locations. An intrathyroidal parathyroid gland can be localized with MIBI scans; however, ultrasound is ideal in assisting the surgeon to determine if the abnormality is indeed parathyroid tissue (Fig. 25.5).





Fig. 25.15
Transverse ultrasound view of a superior right-sided parathyroid adenoma (arrow)

Fig. 25.16
Longitudinal ultrasound view of a superior right-sided parathyroid adenoma (arrow)

Fig. 25.17
(a) Longitudinal ultrasound view showing a superior parathyroid (arrow) above the upper right thyroid lobe with a single feeding vessel. (b) Transverse CT scan in arterial phase of the same parathyroid (arrow). (c) Coronal CT scan in arterial phase of the same right superior parathyroid (arrow)

Fig. 25.18
(a) Transverse ultrasound view of a left superior parathyroid . Notice its posterior location near the esophagus (arrow). (b) Planar dual phase Sestamibi scan showing the lower cervical location of the same superior parathyroid (arrow). (c) Transverse view of a CT scan showing the posterior location by the esophagus of the same superior left sided parathyroid (arrow)
25.4.3 Inferior Parathyroid Anatomic and Imaging Location
The possible locations of the inferior parathyroid are caudad, posterior, or lateral to the lower thyroid pole (69 %) (Figs. 25.8 and 25.9), in the thyro-thymic ligament or thymic tongue (26 %) (Fig. 25.13a, b), superior to the superior parathyroid gland as an undescended inferior parathyroid (<1 %), in the mediastinal thymus or in the mediastinum outside the thymus (2 %) [28, 29, 34]. On ultrasound an inferior gland is usually located in a lower and generally more anterior cervical location when compared to a superior gland [38]. Many times, it appears to protrude out of the lower pole of the thyroid (Figs. 25.13 and 25.19) or sometimes it is “tucked” under the lobe (Fig. 25.20). An extremely rare deep mediastinal parathyroid would be impossible to image with US but 4DCT and and/or SPECT/CT MIBI are usually successful. An inferior parathyroid that failed to descend will remain with a variable amount of thymic tissue high in the neck and the sonographer should always image the area near the hyoid bone, carotid bifurcation, and the submandibular gland to find such glands [39, 40].