18 Ultrasound Assessment During and after carotid and Peripheral Intervention
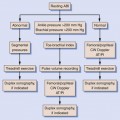
The application of vascular laboratory testing after peripheral arterial intervention can improve procedural and patient outcomes.1–6 Clinical efficacy requires use of appropriate testing methods and interpretation criteria. Duplex ultrasound imaging alone or in conjunction with extremity systolic blood pressure measurements provides accurate diagnostics to identify both technical problems and occlusive lesions that can occur following open surgical bypass or endovascular therapy. Duplex testing is portable and thus suitable for intraprocedural assessment with the goal of testing to improve the technical precision of the arterial intervention. Repair site abnormalities (stenosis, kinks, dissection, thrombus, low-volume flow) interfere with normal functional patency and contribute to early failure.3–8 Routine duplex testing has demonstrated residual stenosis is a common finding following percutaneous transluminal angioplasty (PTA; 5%-25%) as well as infrainguinal vein bypass (10%-15%).1,7–10 When these duplex-detected lesions are identified and promptly revised, the incidence of arterial repair failure is low (1%-2%), which contributes to cost-effective patient care.
In the outpatient setting, duplex testing is the cornerstone of a vascular laboratory–based surveillance program.3,4 Serial testing is performed; initially to confirm that the repair simulates “normal” arterial blood flow, and thereafter to detect developing stenosis caused by myointimal hyperplasia (a common mode of arterial repair failure) or to identify atherosclerotic disease progression. Repeat intervention based on threshold duplex velocity spectra criteria has been shown to increase long-term patency compared to a clinical follow-up regimen that relies on a recurrence of symptoms or signs of peripheral arterial occlusive disease.5
Intraprocedural Duplex Ultrasound Assessment
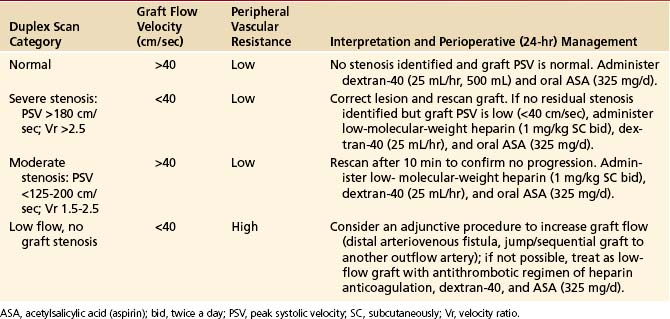
Assessment of an arterial intervention by inspection, pulse palpation, and continuous-wave, handheld Doppler signal analysis is safe and easy to perform but lacks diagnostic sensitivity to identify moderate to severe stenosis or intraluminal defects (dissection, intimal flaps, and thrombus). Although fluoroscopic digital subtraction angiography (DSA) is considered a “gold standard” for arterial repair assessment, the technique is invasive, requiring arterial puncture, radiation exposure, and contrast-induced toxicity. Study interpretation even with multiple imaging runs is prone to false-negative errors.11–13 Duplex testing has emerged as a preferred intraprocedural diagnostic technique since it provides real-time, high-resolution vessel imaging coupled with physiologic information using pulsed-Doppler velocity spectra analysis. Essentially all open and endovascular extremity procedures capable of being insonated with a transducer (either placed directly over the repair or transcutaneous) can be evaluated for technical adequacy using similar interpretation criteria (Table 18-1). Endovascular interventions of the extracranial carotid, subclavian, renal, or mesenteric arteries should be assessed using either DSA alone, with catheter pullback systolic pressure measurement to verify a gradient of less than 10 mm Hg, or intravascular ultrasound. For open arterial surgical repairs, (carotid endarterectomy [CEA], renal/visceral artery bypass or endarterectomy, extremity arterial bypass), intraoperative duplex scanning is easy to perform and interpret using imaging and velocity spectra criteria associated with high diagnostic accuracy (sensitivity and negative predictive value rates of >90%). Procedural duplex imaging can be completed within 5 to 10 minutes and is more convenient than completion arteriography.1,2,14
TABLE 18-1 Diagnostic Methods and “Normal” Threshold Criteria for Commonly Used for Intraprocedural Assessments of Surgical and Endovascular Peripheral Arterial Reconstructions
Reluctance to adopt routine intraprocedural duplex assessment is based on the false belief that testing is difficult to perform and interpret, whereas clinical assessment and arteriography provide equivalent diagnostic accuracy. Intraoperative duplex assessment of infrainguinal vein bypass doubles the immediate revision rate compared to completion DSA, and early (30-day) thrombosis/revision rate is halved. The recommended velocity criteria threshold for repair of a duplex-detected stenosis varies, but a peak systolic velocity (PSV) greater than 150 to 180 cm/sec associated with a velocity ratio (Vr) greater than 2 across the imaged stenosis has been shown to predict both lower extremity bypass and PTA failure. Revision rates based on duplex testing are higher with infrainguinal bypass to an infrageniculate artery (17%), when arm (27%) versus saphenous (15%) vein conduit is used, and when PTA is performed by atherectomy (20%) versus balloon (15%) or stent-angioplasty (5%).15,16
Infrainguinal Bypass Assessment
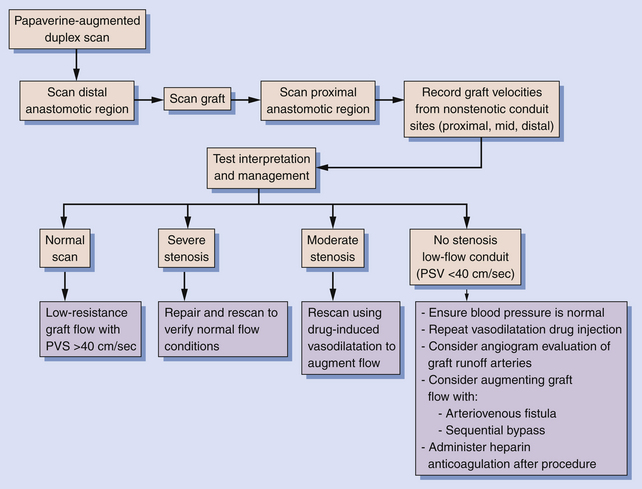
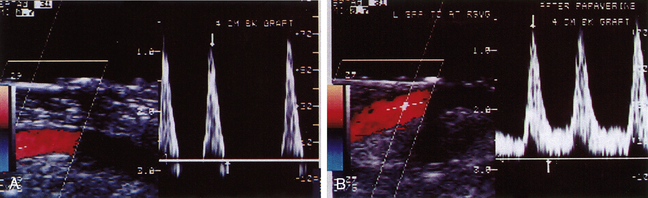
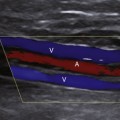
The intraoperative scanning algorithm relies on real-time color Doppler imaging to identify lumen stenosis and sites of disturbed, turbulent flow.10,17 The entire bypass should be imaged to the extent possible beginning at the distal anastomotic region and then proceeding proximally (Figure 18-1). Before duplex scanning, papaverine hydrochloride (30-60 mg) is administered (via a 27-gauge needle into the conduit) to vasodilate the runoff arterial bed and augment graft blood flow. This technique, termed papaverine-augmented duplex scanning, improves diagnostic sensitivity for the detection and grading of residual stenosis severity. Use of a vasodilator drug is also useful to confirm the presence of low resistance flow pattern in distal bypass graft and runoff arteries (Figure 18-2)—a hemodynamic characteristic associated with successful lower limb bypass grafting. Pulsed Doppler velocity spectra are recorded using a Doppler angle of 60 degrees relative to the vessel wall and the sample volume placed in the center stream of flow.
Based on the duplex scan findings, the repair is classified as normal (no stenosis, low resistance graft flow with PSV >40 cm/sec), residual stenosis (moderate, severe), or a low-flow condition is present (Table 18-2). The duplex criteria for a greater than 50% stenosis include color Doppler imaging of an anatomic defect with disturbed color flow pattern and a PSV greater than 180 cm/sec. The PSV ratio across the stenosis should be greater than or equal to 2.5—a level of hemodynamic abnormality that is clinically significant and warrants correction, or at the very least additional evaluation by DSA. An elevated PSV in the range of 125 to 180 cm/sec may be recorded from small-diameter (<3 mm) vessels and should not be considered abnormal if Vr is less than 2.
TABLE 18-2 Interpretation and Recommended Management Based on Intraoperative Duplex Ultrasound Assessment of Infrainguinal Vein Bypasses
A high-resistance waveform (antegrade flow only during systole) in the distal bypass graft is abnormal and when associated with a low systolic flow velocity (PSV ≤40 cm/sec) indicates a “low-flow conduit” and is predictive of early graft failure. In these instances, a careful evaluation for residual proximal or distal occlusive disease should be undertaken. Depending on angiographic and repeat papaverine-augmented duplex scan findings, procedures to augment graft flow, such as construction of a distal arteriovenous fistula or bypass to a second outflow artery, can be considered.
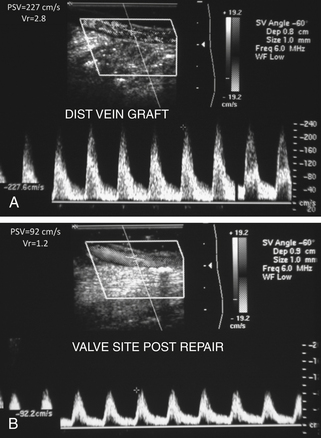
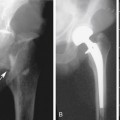
The entire infrainguinal bypass should be imaged for anatomic and flow abnormalities, especially if the bypass technique utilized the in situ saphenous vein with valvulotome vein leaflet lysis. A vein valve site with velocity spectra indicating stenosis (PSV >180 cm/sec; Vr >2.5) should be revised by repeat valve lysis (Figure 18-3). Duplex scanning can also be used to locate patent vein side-branches for ligation. In general, the duplex assessment of prosthetic bypass grafts is limited to imaging of anastomotic sites, since ultrasound insonation of grafts constructed of polytetrafluoroethylene (PTFE) is attenuated by air in the graft wall.
Intragraft platelet thrombus formation is an abnormality that develops in 3% of bypass grafting procedures and has the duplex features of high-grade stenosis (PSV >300 cm/sec) with mobile lumen thrombus seen on real-time B-mode imaging. This lesion is best treated by replacement of the involved vein graft segment, perfusion of the distal graft, and runoff with a thrombolytic agent, followed by reimaging the entire repair for residual flow abnormality as well as the adequacy of graft flow velocity (GFV).
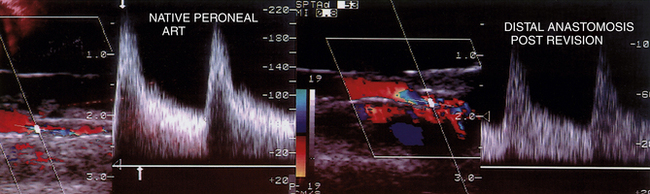
Graft or anastomotic sites with velocity spectra of a moderate stenosis (PSV 125-200 cm/sec; Vr 1.5-2.5) should be carefully imaged (Figure 18-4) for any lumen defects (thrombus, stricture, valve cusp) and rescanned after drug-induced vasodilatation to verify PSV at the site remains less than 180 cm/sec. When elevated velocity spectra (PSV >180 cm/sec) are recorded in an outflow tibial artery but the Vr is less than 2.5 compared to the distal anastomosis PSV, spasm or hyperemic flow is likely the cause and revision is not required. Whenever a low-PSV graft velocity or moderate stenosis is confirmed on the intraoperative study but left unrepaired, a predischarge duplex scan is recommended to verify presence of normal graft hemodynamics versus a persistent residual stenosis or low-flow condition.
A study of 626 consecutive infrainguinal vein bypasses found intraoperative duplex findings predicted outcome (Table 18-3).10 A study interpreted as “normal” had a bypass graft failure rate of 1% at 30 days and 1.5% from 30 to 90 days. If moderate stenosis was identified and not repaired, the incidence of graft thrombosis was 8% at 30 days and the 90-day graft revision rate was 30%. The finding of low graft flow (PSV <40 cm/sec) with no identified graft abnormality was present in only 2% of all bypasses studied, but 5 (38%) of 13 bypasses failed within 90 days. These data indicate that residual duplex-identified defects and low graft flow are risk factors for thrombosis as well as the development of graft stenosis. Intraoperative duplex assessment produced similar primary patency rates at 90 days relative to saphenous vein grafting technique: in situ bypass (94%), nonreversed translocated bypass (94%), and reversed saphenous vein bypass (89%); but lower for arm vein bypasses (82.5%, P <0.01). The application of both intraoperative and predischarge duplex assessment with subsequent revision as necessary resulted in a secondary graft patency of 99.4% at 30 days and 98.8% at 90 days. The observed 15% intraoperative revision rate and a low (2.5%) 90-day failure/revision rate provide the rationale for routine duplex assessment to enhance early outcomes after infrainguinal vein bypass.
TABLE 18-3 Results of Intraoperative Duplex Assessment of Infrainguinal Vein Bypass
| Incidence of Graft Revision | Percent Revised |
|---|---|
| Outflow Artery | |
| Above-knee popliteal | 13 |
| Below-knee popliteal | 16 |
| Anterior tibial | 20 |
| Posterior tibial | 12 |
| Peroneal | 15 |
| Pedal | 17 |
| Bypass Grafting Technique | |
| In situ saphenous vein bypass | 16 |
| Reversed saphenous vein bypass | 10 |
| Nonreversed, translocated saphenous vein bypass | 13 |
| Arm vein bypass | 27 |
| Site of Graft Problem Repaired | |
| Percent of Total Lesions Repaired | |
| Inflow artery | 8 |
| Proximal anastomotic region | 7 |
| Venous conduit | 59 |
| Distal anastomotic region | 26 |
Duplex-Monitored Peripheral Angioplasty
The use of duplex ultrasound to perform and assess an endovascular procedure is a novel but proven application. Angiographic criteria for grading angioplasty site stenosis using diameter-reduction (DR) criteria are inaccurate due to presence of plaque dissection, and the absence of multiplanar image interpretation. Duplex testing is more precise in detection of PTA site stenosis. Successful endovascular therapy of femoropopliteal occlusive disease by balloon PTA (i.e., <30% residual angiogram stenosis) had duplex findings of greater than 50% stenosis (PSV >180 cm/sec; Vr >2) in 20% of limbs.6,14 By life-table analysis, a greater than 50% duplex-detected PTA stenosis was associated with only a 15% 1-year clinical success rate compared to 84% stenosis-free patency when the postprocedural scan was normal.
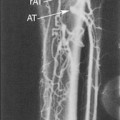
Duplex scanning has been used as the sole imaging technique to perform peripheral endovascular procedures (lower limb, dialysis access). The occlusive lesion to be treated is scanned before PTA to verify its severity (PSV, velocity ratio across the stenosis) and to confirm that the site can be interrogated by duplex ultrasound (Figure 18-5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree