31 Ultrasound Assessment of Native Renal Vessels
Anatomy
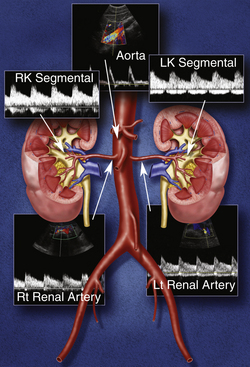
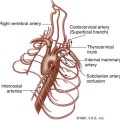
Each kidney receives its arterial supply from one or more renal arteries. The renal arteries arise from the proximal abdominal aorta just below the origin of the superior mesenteric artery, which serves as a reference point (Figure 31-1). The right renal artery arises at an anterolateral location and passes posterior to the inferior vena cava (IVC). It is the only major vessel posterior to the IVC. The left renal artery generally arises from the lateral or posterolateral aspect of the aorta. Anterior to each renal artery runs a corresponding renal vein. Both vessels course anterior to the renal pelvis before entering the medial aspect of the renal hilum. The left renal vein lies between the superior mesenteric artery and the aorta (as opposed to the splenic vein, which lies anterior to the superior mesenteric artery). One of the most common anatomic variants of the renal venous system is a circumaortic left renal vein, in which one of the limbs of the left renal vein courses anterior to the aorta and another one runs posterior to it.
The right kidney is relatively inferior in its position, which explains a long downward course of the right renal artery, traversing behind the IVC and right renal vein. The left renal artery, on the other hand, arises below the right renal artery from the aorta and is more horizontally oriented. It has a direct upward course to the more superiorly positioned left kidney. Duplicate main renal arteries and polar accessory renal arteries occur in approximately 12% to 22% of patients.1–8 Small accessory renal arteries may arise from the aorta or the iliac arteries and usually go unrecognized with ultrasound. Even duplicated main renal arteries may be overlooked sonographically.1–5,9–15
Principles of Examination
Doppler ultrasound evaluation of the renal arteries is one of the most challenging tests to perform given the small size of the renal vessels, their depth, and variation in anatomy. It requires knowledge of the local anatomy, normal waveform physiology, and image optimization. With a little patience and experience, however, a sonographer can become adept at this study and perform the examination in a reasonable period of time. Literature reports indicate that as many as 95% of main renal arteries can be adequately examined in adult patients.9–11,14,16 The key to the renal Doppler examination is accurate demonstration of the vascular anatomy. This requires an understanding of renal vascular anatomy, as well as the ability to recognize normal and abnormal Doppler waveforms.
Technique
The study is performed using 2.5- to 5-MHz curved array transducers for adequate depth of penetration to visualize the abdominal aorta and its major branches: celiac, mesenteric, and renal arteries. Color flow imaging is an integral component of renal artery ultrasound examination. Color flow imaging is used to demonstrate patent renal arteries and detect flow disturbances that indicate stenosis. However, when used alone, this modality may give a false impression of renal artery stenosis, because atherosclerotic plaques can cause flow disturbances in vessels that are not significantly stenotic. A low pulse repetition frequency (PRF) setting may also produce an aliased signal in an area of normal velocity. Pulsed Doppler spectral analysis must be used in conjunction with color flow imaging, as it provides quantitative information through the measurement of blood flow velocity in the renal vessels.
Protocol
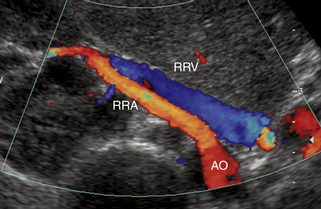
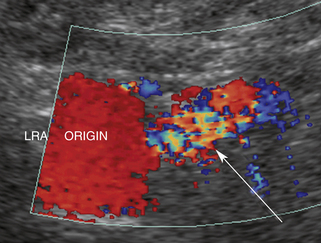
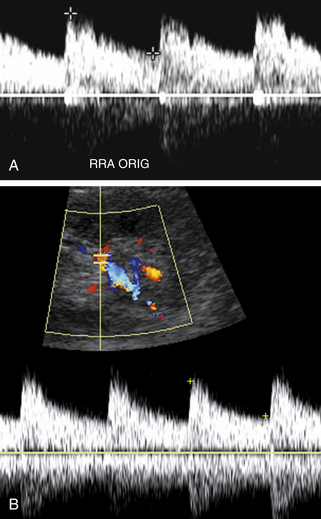
Our protocol for the evaluation of renal arterial disease includes the direct examination of both renal arteries as well as sampling of the segmental branches in both renal hila. When possible, we locate the origin of the renal arteries on transverse images of the aorta using an anterior transducer approach.17 We begin at the celiac axis or the superior mesenteric artery, because these are easily located, and move slightly caudad along the aorta until the origin of each renal artery is seen. The right renal artery is often easier to identify than the left with this approach and is relatively easy to follow to the renal hilum (Figure 31-2). The left renal artery is harder to follow all the way to the kidney from an anterior approach. The left renal artery may be better seen by positioning the patient in a right lateral decubitus position and scanning from a left posterolateral transducer approach,18 using the left kidney as an acoustic window (Figure 31-3). An analogous approach can be used to visualize the distal right renal artery and its branches, with the patient in a left lateral decubitus position. In children, both renal arteries can sometimes be viewed simultaneously from a coronal approach through the left kidney. Transverse and sagittal sweeps of the abdominal aorta and kidneys are performed to identify duplicate renal arteries. These arteries may arise from the inferior aorta or iliac arteries and can be followed to the renal hilum or either pole of the kidney (Figure 31-4).
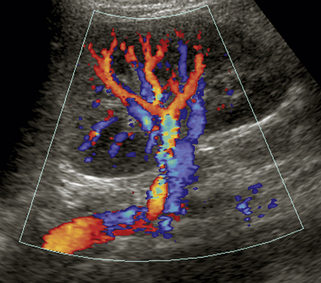
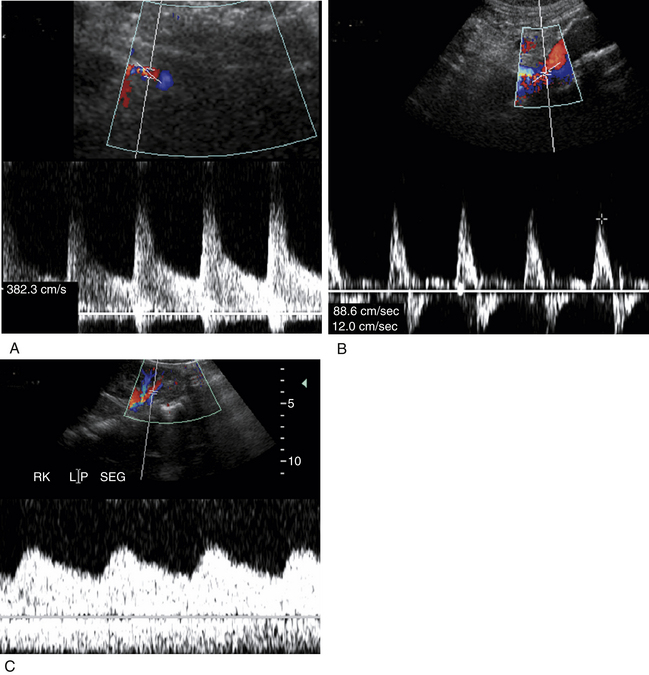
Each renal artery should be examined with color flow imaging from its origin to the hilum of the kidney, including the main hilar branches. Look for areas of high-velocity flow, indicated by color shifts or aliasing, as well as turbulence-related flow disturbances, as these may be related to stenosis (Figure 31-5). Interrogate these areas with spectral Doppler analysis. We routinely obtain PSV measurements from the origin, proximal, mid, and distal segments of each renal artery. A small sample volume (1.5-2.0 mm), and an angle of insonation of 60 degrees or less are used. Finally, waveforms are also obtained from the segmental arteries in the upper, mid, and lower poles of each kidney. Thus, at least seven waveforms are captured from each side. It is important to obtain clean, crisp waveforms with well-defined borders for analysis. This is accomplished by adjusting the spectral display so that the waveforms are large and easily measured.19 This allows the examiner to readily determine the PSV, acceleration time or index, and the resistivity index (RI). The RI is the PSV minus the end-diastolic velocity, divided by the PSV. (The RI may be elevated in numerous conditions, including parenchymal renal disease, acute tubular necrosis, renal vein thrombosis, and urinary tract obstruction.)
The normal PSV range in adult renal arteries is 60 to 100 cm/sec. Normal renal artery waveforms demonstrate a rapid systolic upstroke with persistent forward flow in diastole (low-resistance bed) (Figure 31-6). An early systolic compliance peak (ESP) or notch may be seen in some patients.
Our philosophy on renal artery duplex ultrasound examination is quite pragmatic. We limit the amount of time allotted for our renal Doppler studies. In our experience, a complete renal artery Doppler examination can be performed in as little as 20 minutes. We never exceed 60 minutes. Experienced examiners can assess a patient quickly and determine if the study can be completed in a timely manner. Studies on difficult patients who cannot cooperate or are not “sonogenic” are aborted promptly, and an alternative study is recommended for further evaluation. It is also important to recognize that atherosclerotic renal artery disease is far and away the most common etiology of significant renal artery stenosis, and these lesions occur at the origin and proximal segments of the renal artery. We pay close attention to these segments in our older adult patients who are apt to have atherosclerotic obstructive lesions.10,14 In younger adults, it is more important to see the entire renal artery, as these patients are more likely to have fibromuscular hyperplasia, which can affect the distal renal artery or the segmental branches.13,20
Vascular Disorders
There are a variety of renal vascular disorders that affect the arteries and veins of the kidneys. These conditions may cause damage to the kidneys, renal failure, and/or hypertension. The most common vascular conditions affecting the renal arteries are renal artery stenosis (due to atherosclerosis or fibromuscular dysplasia [FMD]), renal artery occlusion, and renal artery aneurysm. Renal vein thrombosis can be seen with hypercoagulable states, malignancy (tumor thrombus), or propagation of clot from the IVC. Other renal vascular pathologies include arteriovenous fistula (AVF), vasculitis, and pseudoaneurysm.
Renal Artery Stenosis
Stenosis, or occlusion of a main renal artery or a duplicated renal artery, may cause renal ischemia, which in turn triggers the renin-angiotensin mechanism and causes hypertension. Renal artery stenosis can also cause or contribute to renal insufficiency by inducing renal parenchymal damage. The threshold level of renal artery stenosis that produces hypertension or ischemic damage is uncertain and probably varies from one patient to another. Studies suggest that ischemic nephropathy may be responsible for 5% to 22% of advanced renal disease in all patients older than 50 years.21 From a hemodynamic perspective, renal artery obstruction is considered hemodynamically significant (or flow reducing) when the lumen diameter is narrowed by 50% to 60%.
It is estimated that 10% of the U.S. population has hypertension, and 3% to 5% of this group has renal arterial disease.1,22 Although the latter percentages are small, renal artery disease represents the most common correctable cause of hypertension.22 More recently, clinical interest has focused on the potential role of renal ischemia in the etiology of chronic renal insufficiency.23,24 Once again, the potential correctability of renal artery stenosis has been stressed. Few kidney diseases can be cured, and it is understandable that clinicians should be keenly interested in a potentially curable disorder such as renal artery stenosis. Does this mean, however, that we should seek to diagnose renal artery disease in every patient with hypertension or renal insufficiency? To do so could be expensive and not cost-effective.25 Furthermore, intervention for renal artery disease may be risky (e.g., arterial occlusion or rupture) and is not always successful. Considering these points, we believe that renal artery stenosis should be sought in the following groups of patients: (1) young patients with severe hypertension; (2) patients with rapidly accelerating hypertension or malignant hypertension; (3) patients with hypertension that is difficult to control despite a suitable treatment program; (4) patients with concomitant hypertension and deteriorating renal function; and (5) patients with renal insufficiency and discrepant kidney size (implying renal artery stenosis).1,22–25
Doppler Renal Artery Evaluation
1. The principal ultrasound criterion for renal artery stenosis is Doppler-detected flow velocity elevation in the stenotic portion of the vessel.* Flow velocity is increased in proportion to the severity of luminal narrowing; therefore, spectral Doppler measurements can be used to approximate stenosis severity. Narrowed areas detected with color flow imaging must be carefully surveyed with the Doppler sample volume to ensure that the maximum flow velocity is identified.
2. Accurate assignment of the Doppler angle is essential for reliable measurement of stenosis-related velocity elevation, and a Doppler-to-vessel angle of 60 degrees or less is mandatory to ensure that velocity information is accurate.
3. Major stenoses are accompanied by poststenotic flow disturbance (turbulence). Although disturbed flow is a useful beacon for the presence of stenosis, it is neither quantitative nor specific. Disturbed flow may occur without significant stenosis. Color bruit artifacts, however, usually indicate a significant flow abnormality.
4. Arterial waveforms within the kidney (segmental or interlobar arteries) may be scrutinized for evidence of damping, which is a downstream manifestation of renal artery stenosis. The most important downstream findings are the absence of an early systolic peak, a prolonged systolic acceleration time, and a reduced acceleration index (tardus-parvus waveform).6,19,26,28–32
Diagnostic Criteria
Normal blood flow in the renal artery and its branches has a low-resistance pattern with a rapid peak to systole and forward flow throughout diastole. The PSV in normal renal arteries ranges from 74 to 127 cm/sec in both adults and children.17,20,33,34 Children tend to have slightly higher velocities than adults.
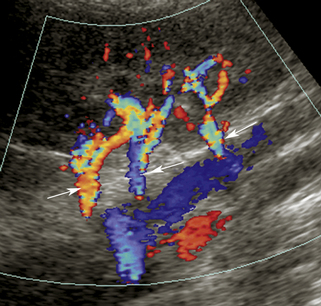
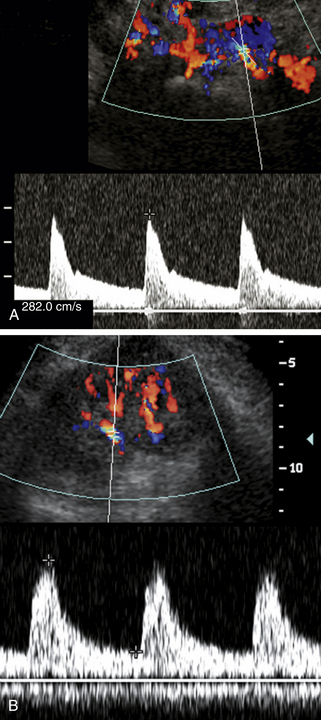
Numerous Doppler velocity criteria have been used to diagnose hemodynamically significant renal artery stenosis (defined generally as 50% to 60% diameter reduction).† The most universally accepted Doppler criteria are (1) PSV in the stenosis of 180 to 200 cm/sec or greater and (2) a renal artery to aortic ratio (RAR) exceeding 3.3 or 3.5.1–3,10–12,16,20 The latter is the ratio of peak systole in the stenotic portion of the renal artery divided by peak systole in the aorta at the renal artery level (Figure 31-7). Some authors have found PSV measurements, used alone, to be more accurate than the RAR.3 In theory, the RAR compensates for hemodynamic variability between patients. Younger patients tend to have higher normal PSV flow in the aorta and branch vessels that can exceed 180 cm/sec without stenosis. Older adult patients, particularly patients with severe cardiac disease and poor cardiac output, may demonstrate lower PSVs, even in regions of stenosis.
Damping of intrarenal arterial signals is also a valuable criterion for diagnosis of renal artery stenosis. Damping is defined numerically with the acceleration index or the acceleration time. Both of these measures reflect the rate of systolic acceleration, which is slower than normal downstream from a hemodynamically significant stenosis. An acceleration index less than 300 cm/ sec2 or an acceleration time exceeding 0.07 second is considered abnormal and suggests a 60% or greater renal artery stenosis.‡ Some authors use an acceleration time of 0.10 or 0.12 second as the cutoff for significant stenosis, which increases specificity.1,2,35
Intrarenal Waveform Assessment
It has long been recognized that renal artery stenosis can cause pulsus tardus and parvus (“tardus parvus”) changes in intrarenal arterial flow signals (see Figure 31-7).36,37 It would be very convenient to simply look for these flow changes in the kidneys and thereby diagnose renal artery stenosis without the arduous task of finding and directly evaluating the renal arteries. Unfortunately, the accuracy of this diagnostic method is questionable. Several literature reports (based on acceleration time, acceleration index, and waveform shape changes) were promising, with sensitivity ranging from 89% to 95% and specificity ranging from 83% to 97% for main renal artery stenoses exceeding 60% or 70% diameter reduction.2,19,28,31 But other literature reports, based on the same Doppler parameters, indicate poor results ranging from moderate accuracy to complete absence of correlation between Doppler and angiographic findings.§ Because of these unfavorable results, this technique for diagnosis of renal artery stenosis has been largely abandoned as the sole diagnostic measure.
The question, then, is why doesn’t intrarenal Doppler work? To begin with, it appears that intrarenal waveform findings are more accurate for high-grade renal artery stenoses exceeding 70% diameter reduction,2,5,30 but even at high levels of stenosis, some patients do not have appreciable waveform damping. This is because the shape of intrarenal arterial waveforms is affected by multiple factors, including the stiffness (compliance) of the arteries, the resistance of the microcirculation, and inflow phenomena, such as renal artery stenosis.39,40 In a patient with generalized arterial stiffness and/or high resistance in the microvasculature from parenchymal renal disease (e.g., diabetes-related nephropathy), the damping effects of a main renal artery stenosis may be obliterated (Figure 31-8). To make matters worse, damped intrarenal waveforms can occasionally be seen in the absence of significant renal artery stenosis in patients with aortic stenosis or aortic occlusion.
It has been suggested that the downstream effects of renal artery stenosis can be diagnosed merely by visual inspection of the shape of the segmental or interlobar Doppler waveforms.19,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree